1. Introduction
2. Material and methods
2.1. Mine tailings
2.2. Bacterial selection and culture
2.3. Bioleaching tests
2.4. X-ray diffraction (XRD)
2.5. Bacterial attachment test
2.6. Statistical analysis
3. Results and discussion
3.1. Arsenic extraction in contact and non-contact systems
3.2. Effects of contact and non-contact mechanism at 0.5% solids
3.3. Effects of contact and non-contact mechanism at 1.0% solids
4. Conclusions
1. Introduction
South Korea has >1000 abandoned mines with stockpiled mine tailings that are rich in heavy metals, including arsenic1). These tailings represent not only an environmental threat but also an economic opportunity, as arsenic is in demand in various industries.
Arsenopyrite mine tailings are the fine-ground residual wastes that remain after beneficiation of a mineral ore. Often, these residual wastes are rich in toxic metals like arsenic (As) and long-term weathering of mine tailings containing arsenic can negatively affect the environment and thus can presents a critical risk in terms of arsenic poisoning. To lower the negative environmental impacts of arsenic or other heavy metals, alternative methods to stabilize metal species are required. Solubilization of arsenic from these residual wastes by bioleaching can be environment friendly, low cost and less labor-intensive process2).
In recent years, bio-leaching has made significant progress in solubilization of metal from low grade resources and secondary wastes3,4). Bioleaching processes exploit the ability of microorganisms to solubilize metals by catalyzing reduction-oxidation reactions for a simplified metallurgical process5). Acidithiobacillus ferrooxidans is among one of the most prominent microorganisms in the biological leaching of (heavy)-metals6,7). These microorganisms predominantly oxidize ferrous (Fe2+) to ferric (Fe3+) ions under aerobic conditions. The strong oxidizing environment resulted from the production of Fe3+ ions in addition to acidic conditions that favor metal solubilization8,9,10,11). The bacteria interact with the mineral substrates by direct contact and non-contact mechanisms, which sometimes cooperate together7,12,13,14). In the contact mechanism, bacteria attach to the substrate surface (e.g., mine tailings) for the solubilization of metal15,16). In the non-contact mechanism, planktonic bacteria oxidize Fe2+ ions to Fe3+ ions in the solution medium. The resulting Fe3+ becomes the oxidizing agent that releases the desired metal ions from its substrate15,16). Following these simple mechanisms, different metals can be solubilize from several types of substrates (e.g., contaminated soils, low-grade resources, residual wastes) as long as Fe2+ is available for bacterial oxidation.
Up to now, no detailed work is observed on the importance of bacterial contact and non-contact mechanism for solubilization of arsenic from indigenous mine tailing. Therefore, to investigate the possibility of improving the arsenic solubilization efficiency of bioleaching, we studied the influences of the bacterial attachment and solid concentration on the arsenic solubilization from mine tailings. The contact and non-contact mechanisms were conducted by installing a partition system with a semi-permeable membrane, which prevents bacterial attachment to the mine tailings’ surface. The experiments were conducted with two solid concentrations (0.5% and 1.0% w/v) with constant temperature, shaking speed, and particle size. These findings will better elucidate the influences of each of the bioleaching mechanisms on arsenic solubilization efficiency from mine tailings and will give an insight into appropriate methodologies development for increasing metal bio-extraction efficiency.
2. Material and methods
2.1. Mine tailings
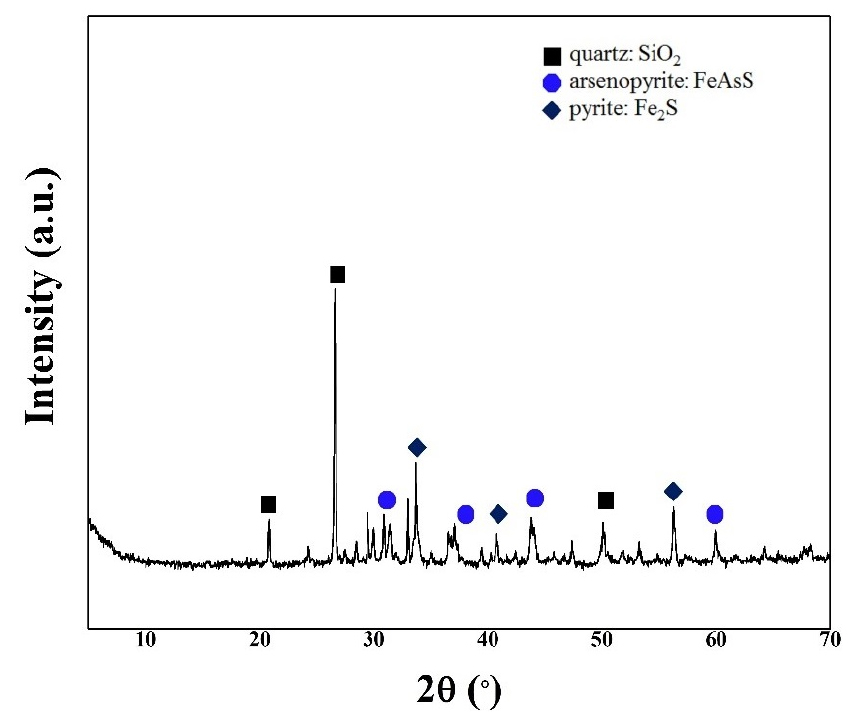
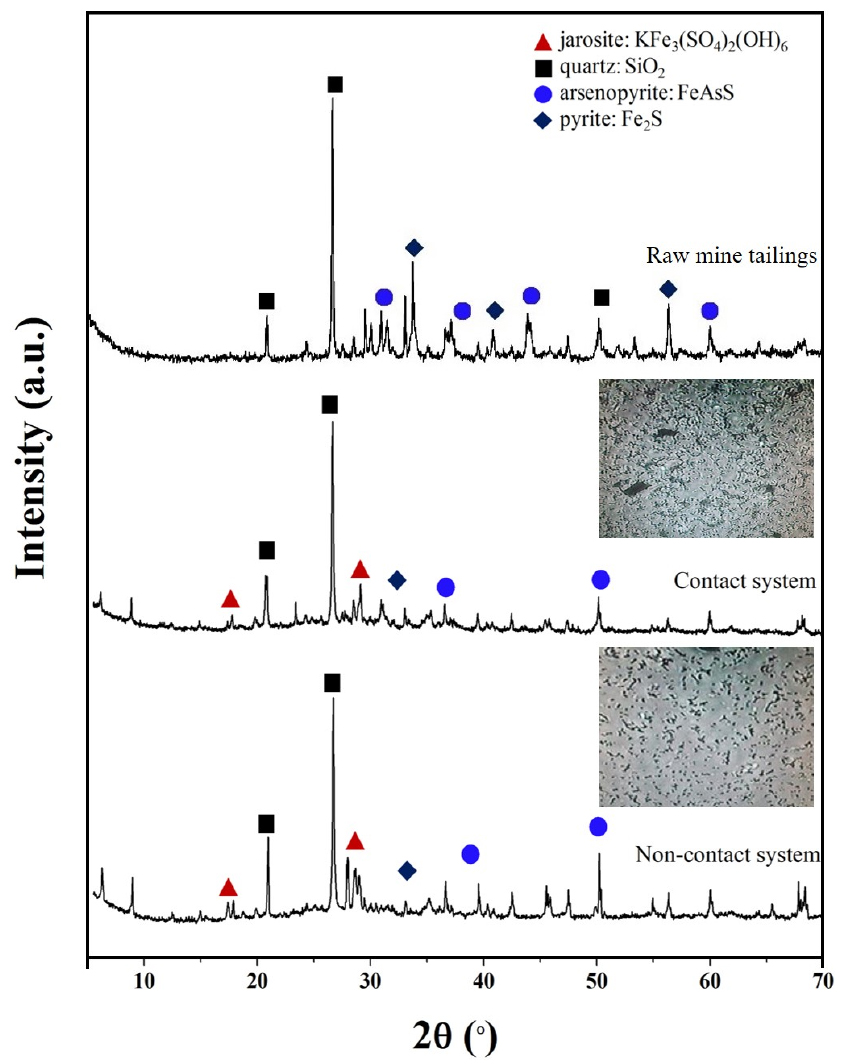
An oxidized mine tailings sample was obtained from the Janggun mine (from a Pb, Zn, and Ag-mining site in Bonghwa, Gyeongsang, South Korea)7). The sample was kindly provided by the Mine Reclamation Corporation. The particle sizes of the mine tailings in the bioleaching tests ranged from 63 to 74 μm (measured by a sieving technique) to determine contact and non-contact mechanisms except size effect. The chemical composition and mineralogy of the mine tailings were determined by inductively coupled plasma (ICP, Optima 7300DV, PerkinElmer) and X-ray diffraction (XRD, Bruker D8 HRXRD, Germany) analyses, respectively. The ICP analysis confirmed the high As content in the mine tailings (53200 mg/kg; see Table 1). Most of the As-associated mineral existed as arsenopyrite (FeAsS) (XRD analysis; see Fig. 1). Additionally, the Fe content in the mine tailing was determined to be 183364 mg/kg, and the presence of pyrite (FeS2) was confirmed. FeS2 is a recognized suitable source of ferrous ions for Acidithiobacillus sp. during bioleaching processes17,18,19).
Table 1.
Physical properties and chemical composition of the mine tailings
| Materials | d50(μm)a | ρa(g cm-3) |
Specific surface area (m2 g-1)b |
Chemical composition (mg/kg) | |||||
| Mine tailing | 73 | 2.65 | 13 | As | Cd | Cu | Zn | Pb | Fe |
| 53200 | 154 | 4966 | 19461 | 9808 | 183364 | ||||
2.2. Bacterial selection and culture
Acidithiobacillus ferrooxidans (KCTC 4515) was obtained from the Korea Research Institute of Bioscience and Biotechnology, South Korea. The culture medium for the bacteria was prepared with reference to DSMZ medium 88220). The initial pH value was adjusted to 1.8 with 10 N H2SO4 (Fisher Scientific), and the speed and temperature of the shaker (SIF-5000R, JEIO TECH, Seoul, Republic of Korea) was fixed at 150 rpm and 30 °C, respectively. The bacterial concentration in the bioleaching medium was determined by a Burker–Turk cell counting chamber (Marienfeld Laboratory Glassware, Germany) under phase-contrast microscopy (ODEO-2003 Triple, IPONACOLOGY). In all bioleaching experiments, the initial bacterial concentration was adjusted to 1.2 × 108 cells mL−1.
2.3. Bioleaching tests
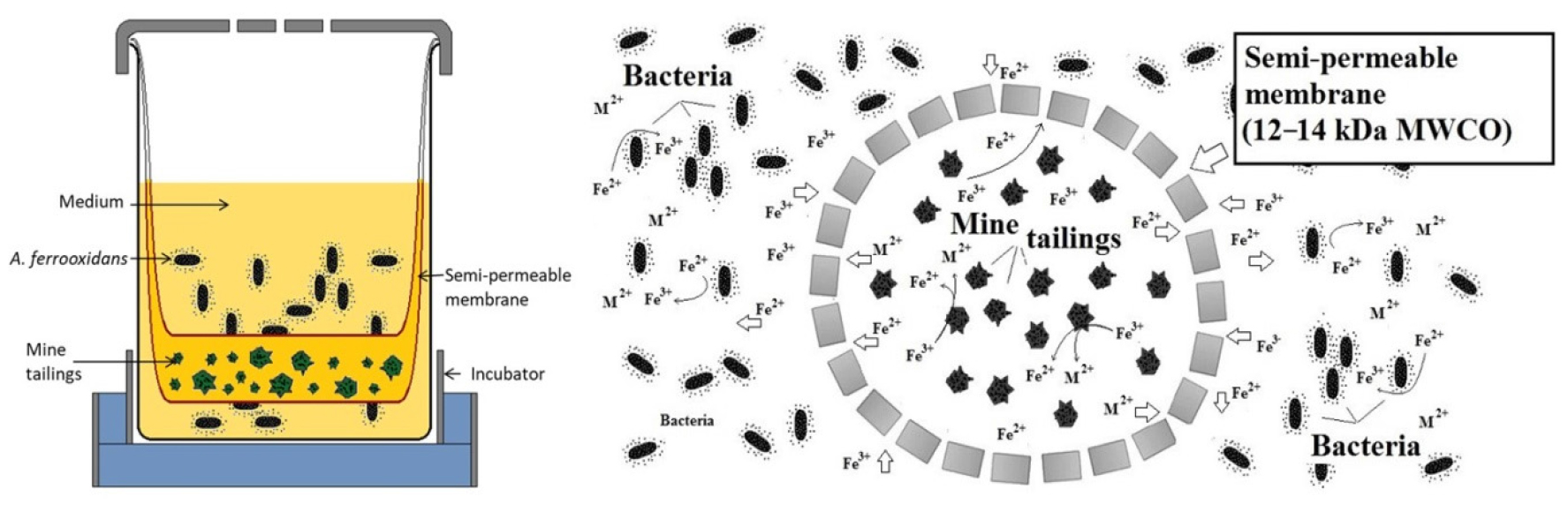
Prior to the bioleaching tests, bacterial cultures of A. ferrooxidans were grown to reach stationary phase (i.e., 48 h). Experiments were carried out in 500 mL beakers at solid mine tailing concentrations of either 0.5% or 1.0%, 30 °C, and 150 rpm. To study the effects of bacterial attachment, A. ferrooxidans was separated from the mine tailings by a partition system21,22) consisted of a semi-permeable membrane with a molecular weight cutoff (MWCO) of 12–14 kDa (Spectra/Por® 4 membrane, Spectrum® laboratories, Inc. CA. USA). The partition system setup (Fig. 2) was designed as proposed by Silva et al. (2015)21). Metal ions were confirmed to pass freely through the membrane, eliminating any possible negative effects on the bioleaching results.
Inside the membrane of the partition system, we pipetted 10 mL of DSMZ medium 882 containing either 1.5 or 3.0 g of solid (corresponding to solid concentrations of 0.5 and 1.0%, respectively). The chamber outside the membrane was seeded with 30 mL of bacterial suspension and 290 mL of DSMZ medium 882. In the non-partitioned system, 30 mL of bacterial suspension and either 1.5 or 3.0 g of mine tailings were added to 300 mL of DSMZ medium 882. The oxidation/reduction potential (ORP), pH, and metal (As and Fe) concentrations were measured daily. After measuring the solution pH (ORION 4STARS, Thermo) and ORP (Hanna Instruments, model 2211), a 2.0-mL aliquot was sampled from the leachate and filtered through a 0.45-μm nylon syringe filter (Corning Incorporated, Corning, Germany). The As and Fe concentrations in the aliquots were determined by ICP analysis. Additionally, the Fe2+ concentration was analyzed by the o-phenanthroline method23), and the Fe3+ concentration was calculated as the difference between the total Fe and Fe2+ contents.
To compare the total Fe and Fe3+ concentration dynamics between the contact and non-contact systems, we re-expressed the total Fe and Fe3+ concentrations as Eqs. (1) and (2), respectively. Here, fT,Fe denotes the relative total Fe concentration at a certain reaction time t, defined as the total Fe concentration CT,Fe at time (t) divided by the maximum total Fe concentration CFe (Max) over the entire reaction time. Similarly, fFe3+ denotes the relative Fe3+ concentration at reaction time t and is defined as the Fe3+ concentration CFe3+ at time (t) divided by CT,Fe (t).
2.4. X-ray diffraction (XRD)
The XRD analysis revealed the mineral forms present in the mine tailings before and after bioleaching tests. The raw mine tailings were ground in a mortar. After the bioleaching experiments, the precipitates were dried at 80 °C and ground for analysis. Powder XRD patterns were collected within the 2θ range of 5°–70° in steps of 0.01°, with a counting time of 1 s step−1. The XRD instrument was a Bruker D8 HRXRD (Germany) with CuKα radiation (λ = 0.154606 nm, 40 kV, 40 mV) and a SolX detector. The spectra were evaluated using the Diffracplus EVA package.
2.5. Bacterial attachment test
The bacterial attachment test determined the extent of initial bacterial attachment to the mine tailings. The attachment test was carried out under the same conditions as the bioleaching process (pH = 1.8, temperature = 30 °C, shaking speed = 150 rpm, initial cell centration = 1.2 × 108 cells mL−1), but without nutrient supplement (as cell growth was undesired) to prevent bacterial growth over time. Note that several studies reported that initial cell adhesion to substrates normally govern biofilm formation24,25,26). The solution ionic strength, which is a recognized critical parameter for cell adhesion, was adjusted with NaCl to 320 mM (the ionic strength of the culture medium for bioleaching). The attachment test was conducted in a 50 mL centrifuge tube (BD FalconTM, USA) with a shaker (WiseShake SHR-1D, Witeg, Germany). The number of cells attached to the mine tailings was determined as the difference between the initial cell concentration and the cell concentration at specific time intervals (0, 40, and 80 min). The maximum time (80 min) is the equilibrium time of cell adhesion, as determined in previous studies27,28). The cell number was determined by a Burker–Turk counting chamber. All tests were triplicated (at least) to ensure reproducibility.
2.6. Statistical analysis
In the figures, mean data are presented with error bars indicating one standard deviation. Differences between mean values were analyzed using Student’s t-test and were considered statistically significant when P < 0.05.
3. Results and discussion
3.1. Arsenic extraction in contact and non-contact systems
Arsenic extraction in non-contact system can represent chemical extractions facilitated by oxidizing agents produced biologically in a two-step bioleaching system without any contact to mine tailing. Solubilization of metals by oxidizing agents produced biologically, like Fe3+, can be summarized in equation 3 as suggested by Sand et al., (2001)29):
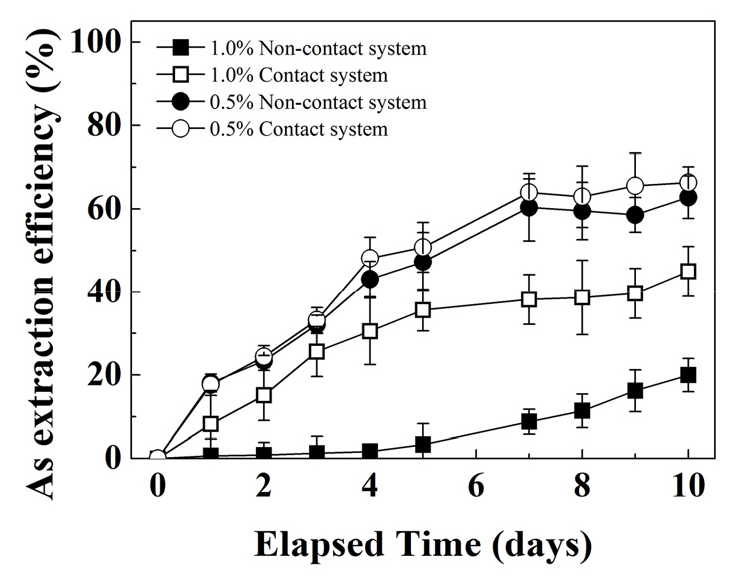
Fig. 3 shows the effect of non-contact mechanism when comparing arsenic extraction in systems with 0.5 and 1.0% solid concentration. Higher arsenic extraction was observed with lower solid concentration.
3.2. Effects of contact and non-contact mechanism at 0.5% solids
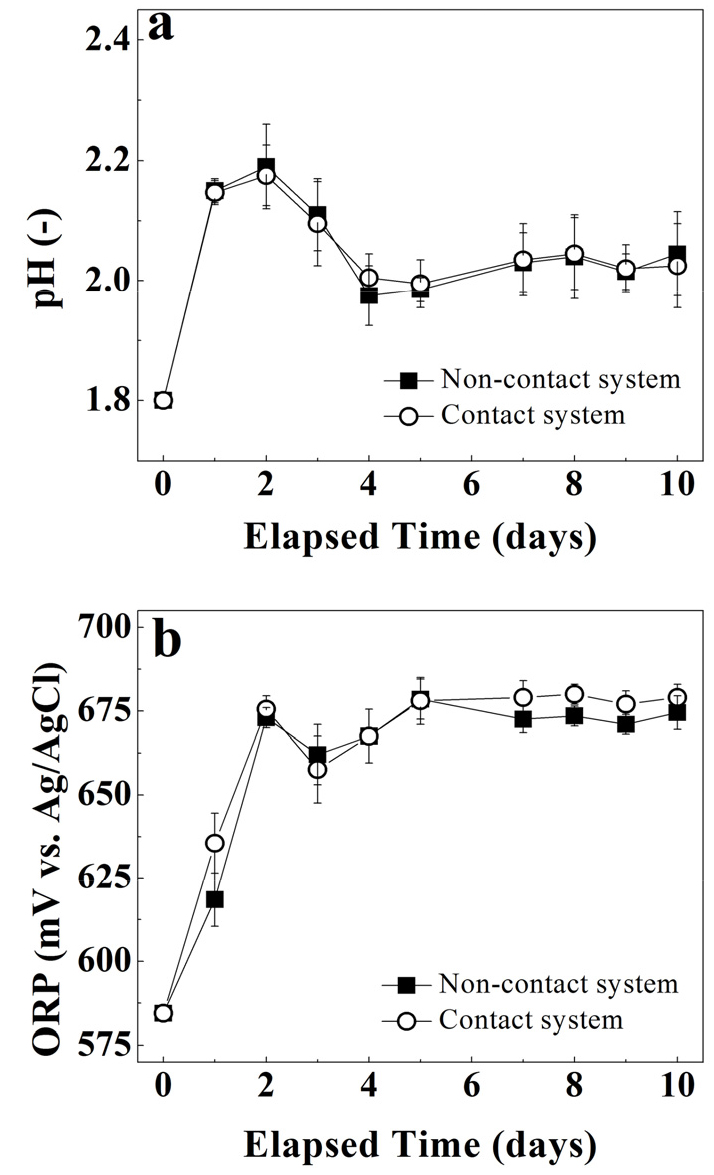
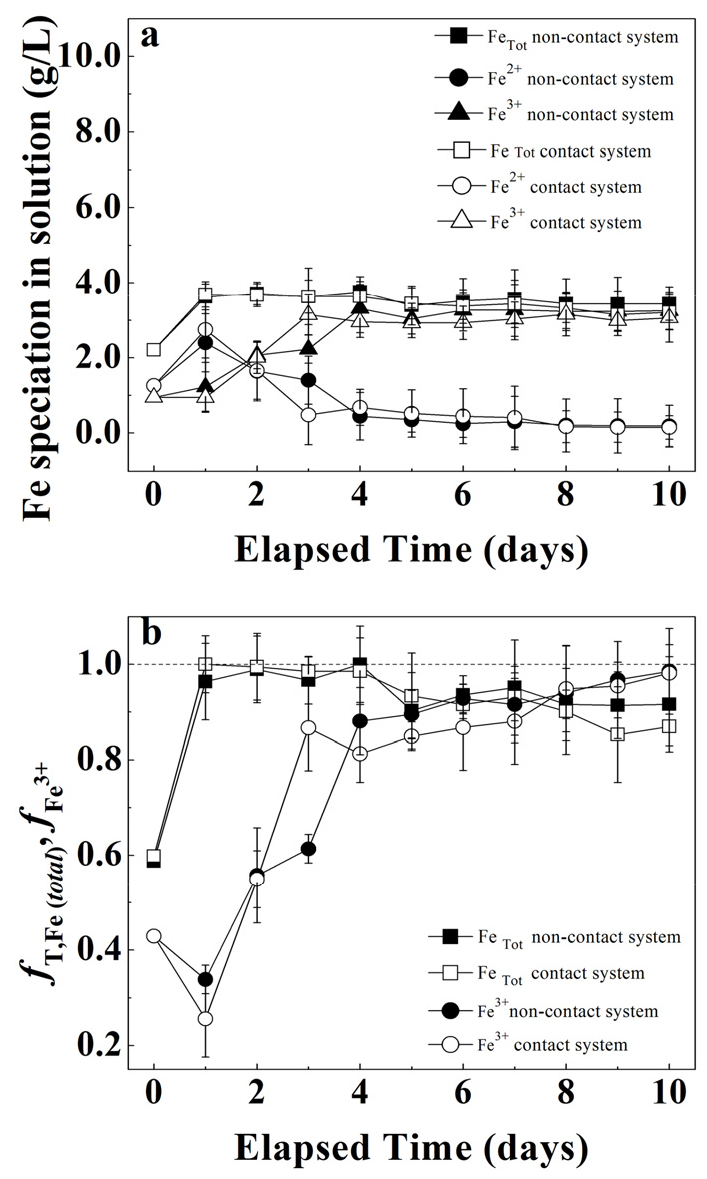
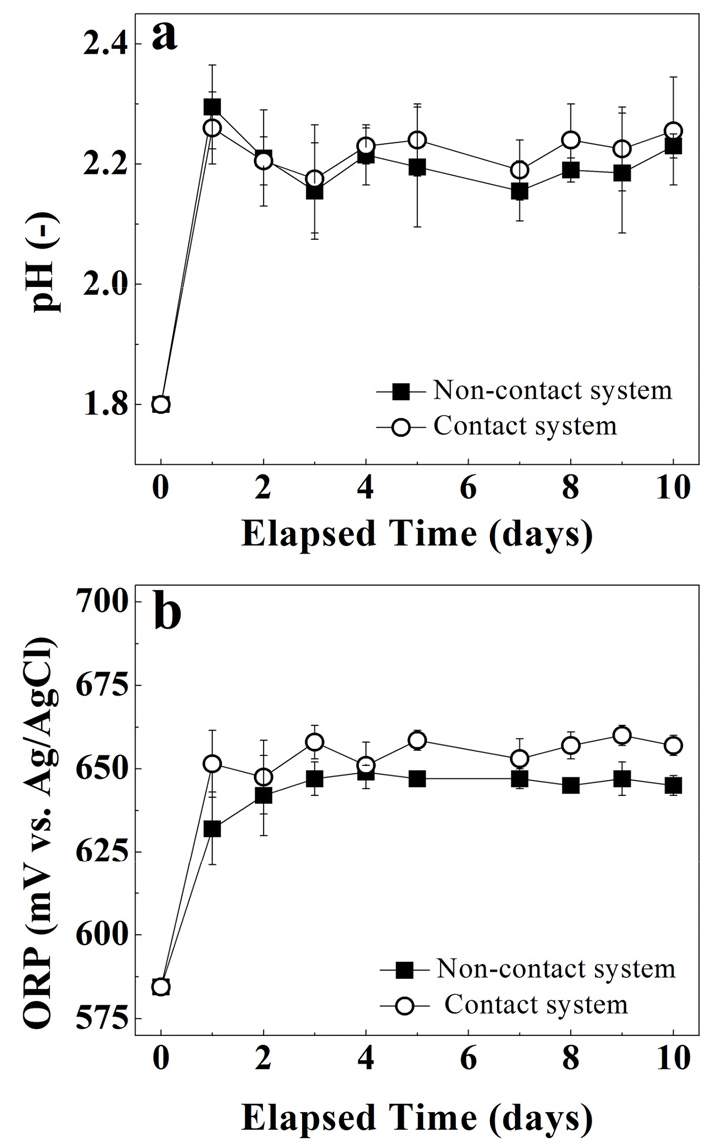
The arsenic solubilization for both contact vs. non-contact system at solid concentrations of 0.5% show overlapping behaviors. Both system’s trends increased linearly, exceeding 60% during the first 7 days of experiments and reached a plateau for the subsequent days (see Fig. 3). The pH profile for both systems also show overlapping behaviors (see Fig. 4a) increasing from 1.8 to 2.2 in the first two days and then decrease to 2.0 after 4 days and remained relatively constant afterwards. Similarly, the redox potential profile (See Fig. 4b) also show similar behaviour for both systems from 580 mV to 675 mV in first 3 days of experiment and then stayed constant until the end of the experiment.
As observed in Fig. 5a & 5b, the Fe speciation behaved similarly for both contact vs. non-contact systems throughout the bioleaching experiment. Fig. 5a shows a rapid decrease in Fe2+ concentration after the first day of experiment indicating its conversion to Fe3+ ions as expressed by equation 4:
In addition, the fT,Fe (Fig. 5b) in both systems rapidly increased throughout the first 24 h of reaction, suggesting a release of Fe from mine tailings as is also reflected by the total Fe trend in Fig. 5a. After the sharp increase in the first 24h, fT,Fe remained relatively stable with a slight decrease at the end of the bioleaching experiment. Contrarily, fFe3+ slowly increased during the first four days of reaction suggesting a slow oxidation of Fe2+ to Fe3+ by bacterial activity. Nevertheless, stable oxidations rates (i.e., above 650 mV vs Ag/AgCl) were observed after the fifth day of reaction (Fig. 4b), together with a depletion of Fe2+ in the system (Fig. 5a) suggesting a higher bacterial activity in both systems.
Thus, we conclude that bacterial attachment do not show significant improvement in arsenic solubilization at solid concentrations of 0.5% solids. Similar Fe concentration trends also suggest that attached bacteria did not increase Fe solubilization from the FeS2 and FeAsS contained in mine tailings. We believe that even if bacteria attached to the surface of mine tailings the amount of 0.5% solid is not enough to sustain a significant increase in the efficiency of arsenic extraction.
3.3. Effects of contact and non-contact mechanism at 1.0% solids
Contact mechanism studied with 1.0% solid concentration showed 75% increase in the As extraction in 10 day bioleaching period (Fig. 3). Interestingly, during the first 5 days, the arsenic extraction in contact system increased linearly reaching values close to 40%, whereas the arsenic extraction was negligible in the non-contact system. However, in the subsequent days the arsenic extraction levelled off while the non-contact system increased linearly reaching a maximum of 19% by the end of the experiment.
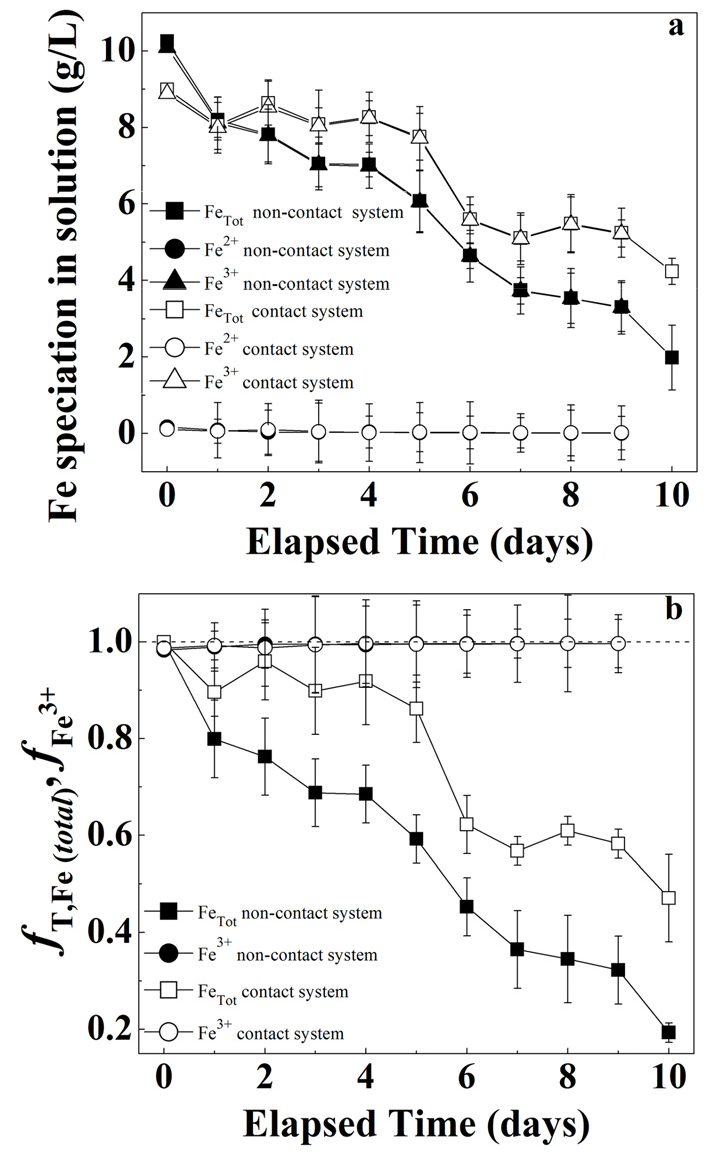
Figs. 6a, 6b, 7a, and 7b represent pH profile, the ORP, the changes in the Fe speciation and relative concentrations of the total Fe and Fe3+ species in contact and non-contact system at 1.0% solid concentration. The pH increased from 1.8 to 2.3 within the first day of bioleaching and then stayed constant over the remaining test period in both systems. The fluctuations in pH measurement among contact vs. non-contact system were within experimental error. Likewise, the ORP followed similar trend (an increase in ORP within first day of bioleaching) and then stayed constant throughout the rest of the experiment reaching different magnitudes 660 mV vs. 640 mV in contact vs. non-contact system, respectively. Higher redox potentials correspond to stronger oxidizing environments. Therefore, a higher ORP in contact system provide indirect evidence of different rates of Fe2+ to Fe3+ oxidation among systems (Equation (4)). Similarly, a higher oxidation rate of Fe2+ to Fe3+ suggest a higher bacterial activity, as it is expressed by equation 429). At the end of the experiment, the difference in ORP trends was found to be statistically significant (P < 0.05) suggesting that Fe3+ was produced at higher rate when there was no barrier between bacteria and mineral substrate.
Contrarily, the total Fe and Fe3+ concentrations in both systems decreased with bioleaching time (Fig. 7a). Notably, the Fe2+ concentration decreased under the detection limits. Thus, it is understood as an “instantaneous” oxidation from Fe2+ to Fe3+ due to bacterial activity in both systems. In contact system, within 1-5 days, total Fe and Fe3+ decreased slightly from 9 g/l to 8 g/l, indicating the availability of Fe3+ to leach arsenic in the solution. This may explain a greater extraction of arsenic within 1-5 days in contact system (See Fig. 3). In next 6-10 days, total Fe and Fe3+ decreased by 50% in contact system (i.e., from 8 g/l to 4 g/l) indicating less availability of Fe3+ in solution. Therefore, it decreased the extraction of arsenic in the solution and reached the plateau observed (see Fig. 3). In contrast, in the non-contact system the total Fe and Fe3+ decreased steadily from 10 g/l to 2 g/l thus showing reduced extraction efficiency.
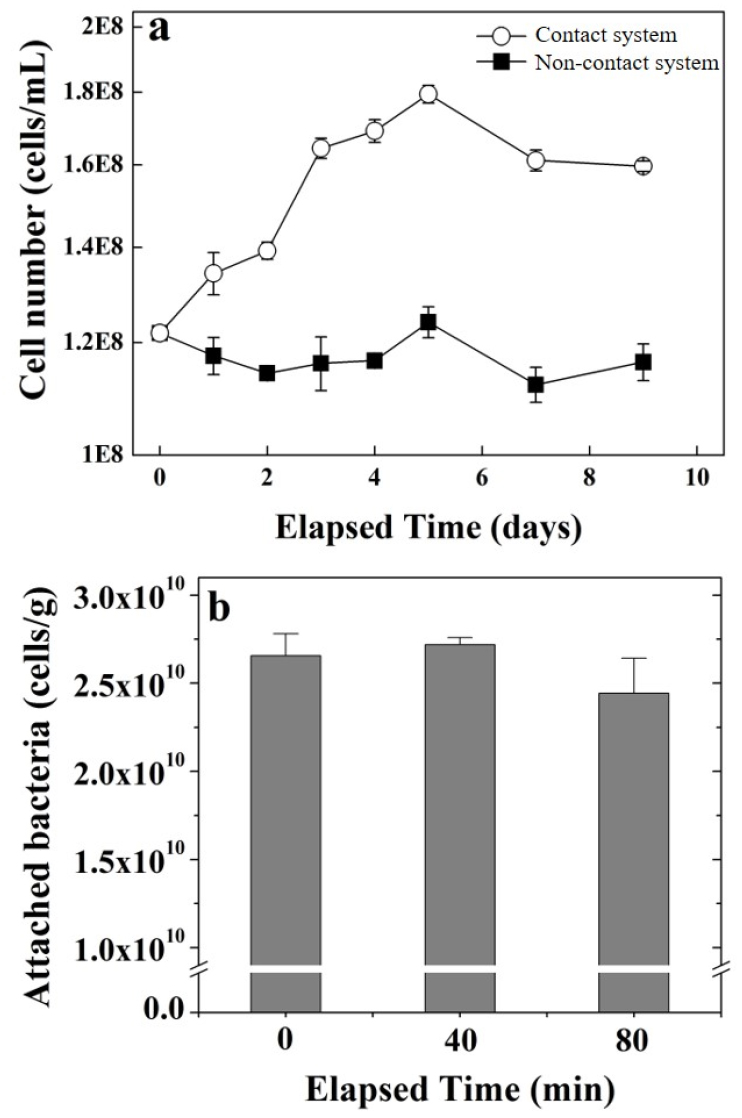
At pH values exceeding 2.2, the decrease in total Fe in bioleaching system has been attributed to the precipitation of Fe3+ as insoluble Fe3+ species like jarosites and iron hydroxides21,30,31). The precipitation largely depends on the Fe3+ concentration, solution pH, ORP, and solid concentration7,30). Therefore, a decreased in total Fe observed at 1.0% w/v solid concentration likely represents the precipitate formation that reduces the Fe availability in solution. This was more prominent in non-contact than contact system. Importantly, Fe3+ can also precipitate with As-associated minerals, possibly reducing the As concentration in solution13,32). Jarosite formation was confirmed by XRD analysis (Fig. 8) that can form a passivation layer on tailing sample and inhibiting the interaction between the metals and the oxidizing agents thus decrease bioleaching of arsenic13). The effect of decreasing the availability of Fe2+ ions on the bacterial population was confirmed in Fig. 9a showing lower bacterial concentrations in non-contact system compared to contact system.
Initial bacterial attachment to the mine tailing surface was confirmed by a cell adhesion test conducted during the first 80 min of reaction. Approximately 2.45 × 1010 cells were attached to each gram of mine tailings (Fig. 9b) accounting to around 88% of the initial cell concentration.
By confirming bacterial attachment, altogether with an improvement of arsenic extraction in contact system, we conclude that non-contact mechanism negatively affects the extraction of arsenic from mine tailings. Decrease in bioleaching efficiency at high solid concentrations can be due to less tolerance of bacterial culture with tailing, oxygen deficiency in liquid media, and toxic effect of heavy metals. However, all of the drawbacks of systems at high concentration can be overcome by bacterial adaptations to high solid concentrations.
4. Conclusions
Current work can be a fundamental step to better understand environment aspects around indigenous mine sites of Korea and to reduce detrimental effect of tailing by proper management prior to dumping/discarding. The bioleaching efficiency of arsenic was less in non-contact system compared to contact system at 1.0% solid concentration. While at 0.5% solid concentration the effect of contact and non-contact mechanism was not so prominent. Interestingly, at 1.0% of concentration solid concentration, the arsenic extraction increased by 75% with contact mechanism. Therefore, we conclude that processes that base the metal extraction solely on oxidizing agents produced biologically on independent reactors, as the so-called two-step or non-contact bioleaching process, are not suitable for the extraction of arsenic from mine tailings. Bacterial adaptations are recommended to further improve overall arsenic extraction efficiency at high solid concentrations.