1. Introduction
2. Experimental
2.1. Materials and chemicals
2.2. Leaching procedure
3. Results and discussion
3.1. Effect of the nature of oxidizing agents
3.2. Effect of HCl concentration
3.3. Effect of sodium chlorate concentration
3.4. Effect of pulp density
3.5. Effect of reaction time
3.6. Effect of temperature
4. Conclusions
1. Introduction
Currently, about 30 kinds of ores containing molybdenum have been found in nature. However, only molybdenite (MoS2) and wulfenite (PbMoO4) are considered to be important ores for the extraction of molybdenum1,2). Due to its high melting point, high strength at high temperatures, high thermal conductivity, and good corrosion resistance, molybdenum is widely used in many materials such as construction steel, stainless steel, and chemicals3,4). Molybdenum is also an element of value to all living organisms because of its functional role as a cofactor in various enzymes of bacteria, plants, and animals5). In addition, molybdenum has also made an important contribution to the green energy industry, where it is used in high-strength steel for automobiles to reduce weight and improve fuel economy and safety. Because of the many important applications in many industries and the increasing use of molybdenum, the recovery of molybdenum from ores and secondary resources is important.
Over the years, many processes for recovering molybdenum from ores have been developed including oxidation roasting6), direct reduction of molybdenite7), chlorination8), and pressure oxidative leaching9), acid leaching10,11,12), hypochlorite leaching13,14,15), and bioleaching16). Cao et al.10) dissolved molybdenum sulfide ores with hydrochloric acid containing sodium chlorate as an oxidizing agent. The leaching percentage of molybdenum was 98% at the following optimum conditions: 240 min, 20% HCl, liquid-to-solid ratio 10:1, stirring speed 500 rpm, 70°C, and the molar ratio of NaClO3 to MoS2 to be 3.2. Lasheen et al.11) investigated the leaching of molybdenum-uranium ore in sulfuric acid solution with hydrogen peroxide as the oxidizing agent under the conditions of 95°C, 0.5 M H2O2, 2.5 M H2SO4, stirring speed 600 rpm, solid-to-liquid ratio 1:14 for 120 min, particle size 74 m and the leaching percentage of molybdenum was about 98.4%. Youcai et al.13) examined the leaching of molybdenum from copper concentrate by sodium hydroxide containing sodium hypochlorite along with agitation speed 500 rpm, 10% NaOH, 8% NaClO, liquid-to-solid ratio 10:1 at 50°C during 4 h. Under these conditions, the extraction of molybdenum was greater than 99.9% and the extraction of copper was less than 0.01%. These methods can recover molybdenum with high efficiency. In general, the addition of an oxidizing agent is necessary to dissolve sulfide ores.
Cao et al.10) extracted about 98% molybdenum from molybdenite concentrate by HCl containing sodium chlorate. However, the effect of oxidizing agents on the extraction of molybdenum was not investigated. In this study, the effect of sodium chlorate as an oxidizing agent on the extraction of molybdenum from the molybdenite ore by hydrochloric acid was examined. Moreover, the effect of other conditions such as pulp density, time, and temperature on the leaching of molybdenum from molybdenite ore was investigated. An optimum condition for the leaching of molybdenite ore was obtained.
2. Experimental
2.1. Materials and chemicals
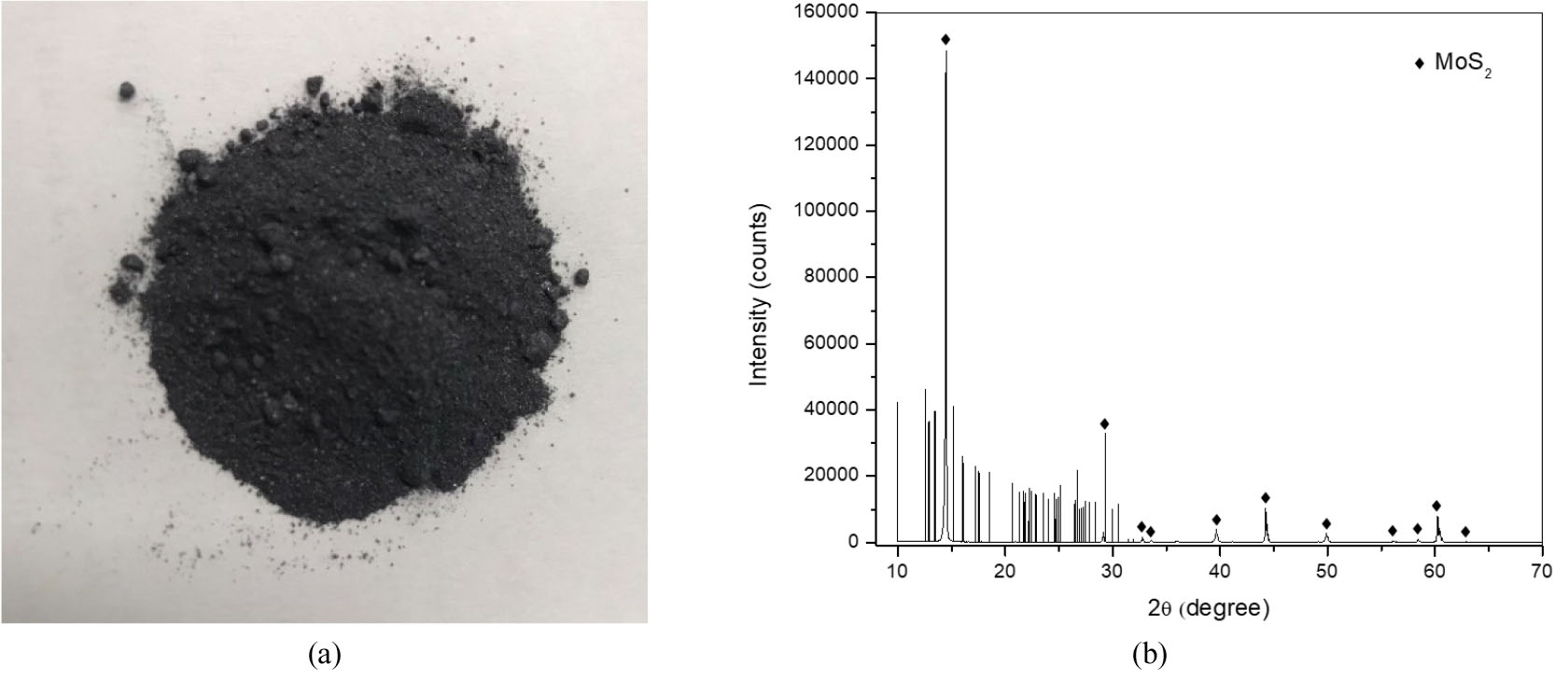
The molybdenite ore employed in this work was provided by a company in Korea. The ore samples were dried in an oven at 60°C for 24 h and then ground to a grain size of 300–500 μm. The ground samples were stored in a glass jar with a lid to avoid oxidation. The chemical composition of the ore samples obtained by X-ray fluorescence (XRF) spectrometer (M1MISTRAL, Bruker, Karlsruhe, Germany) is shown in Table 1. As is shown in Table 1, the predominant component of the ore was molybdenum and a small amount of iron, calcium, and silicon existed in the ore. Therefore, the leaching behavior of these four components (Mo, Fe, Ca, and Si) was investigated in this work. The mineral composition of the molybdenite ore was analyzed by X-ray diffraction (XRD) and the results are shown in Fig. 1.
Table 1.
Chemical composition of the molybdenum ore employed in this work by XRF
Hydrochloric acid (HCl, Daejung Metal & Chemical Co., Ltd., Korea., 35%) was diluted with doubly distilled water to the desired concentrations. Some chemical reagents such as hydrogen peroxide (H2O2, Daejung Chemical. Co), Korea., 30%), sodium hypochlorite (NaClO, Daejung Chemical & Metal Co., Korea, 8%), and sodium chlorate (NaClO3, Daejung Metal & Chemical Co., Ltd., Korea.) were used without any purification.
2.2. Leaching procedure
The leaching experiments were performed in a 250 mL three-necked round-bottom flask with a specified volume of HCl solution. The stirring rate, reaction time, and temperature were controlled by using a magnetic stirrer (WiseStir MSH-20D, Daihan Scientific Co., Seoul, Korea) throughout the reaction. The molybdenite powder was introduced into the reaction flask once the desired temperature of the solution was reached. After the leaching experiments, filter paper (ADVANTEC No. 2, 110 mm, Toyo Roshi Kaisha, Ltd.) was used to separate the residue and filtrate. The concentration of the metal ions in the aqueous solution was determined by using inductively coupled plasma optical emission spectrometers (ICP-OES, Spectro Arcos, Germany) and the leaching percentage (%L) was calculated as follows
where msample and msolution are the mass of the metals in the ore and the filtrate, respectively.
3. Results and discussion
3.1. Effect of the nature of oxidizing agents
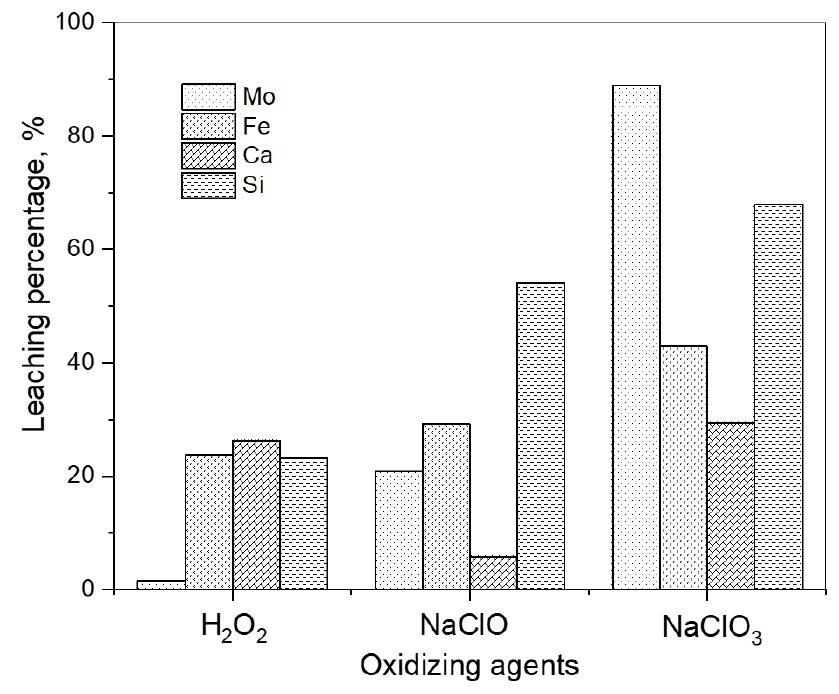
Molybdenite ore is quite chemically inert and relatively unreactive17). Therefore, an oxidizing agent is needed to oxidize the sulfide, which facilitates the dissolution of molybdenite. Three kinds of oxidants such as hydrogen peroxide, sodium hypochlorite, and sodium chlorate were employed in this work. In order to compare the efficiency of these oxidizing agents in the leaching of the molybdenite ore, preliminary leaching experiments were done at the following conditions: 2.0 M HCl, pulp density 20 g/L, stirring speed of 400 rpm, 240 min, and 70°C. In these experiments, the concentration of the three oxidizing agents was fixed at 0.5 M.
Fig. 2 shows that the leaching percentage of the metals by NaClO3 was higher than that by H2O2 and NaClO. Specifically, when NaClO3 was used, the leaching percentage of molybdenum, iron, calcium, and silicon was 88.9%, 42.9%, 29.41%, and 68.0%, respectively. The leaching percentage of molybdenum, iron, calcium, and silicon by NaClO was 20.9%, 29.2%, 5.8%, and 54.1%, respectively. In the case of H2O2, the leaching percentage of molybdenum, iron, calcium, and silicon was 1.5%, 23.7%, 26.3%, and 23.2%, respectively. The standard reduction potentials of H2O2, NaClO, and NaClO3 are shown in Table 211,18). Among the three oxidizing agents, the reduction potential of NaClO3 is the highest, indicating that the oxidizing power of the leaching solution is the strongest. Therefore, further experiments were done by using NaClO3 as an oxidizing agent.
Table 2.
| Haft - reaction | E°, V |
| 2ClO3-(aq) + 12H+ + 10 = Cl2(g) + 6H2O | 1.47 |
| O2(g) + 2H+ + 2 = H2O2(aq) | 0.68 |
| 2ClO-(aq) + 2H2O(aq) + 2 = Cl2(g) + OH- | 0.42 |
| MoS2 + 12H2O = H2MoO4 + 2SO42- + 22H+ + 18 | 1.105 |
3.2. Effect of HCl concentration
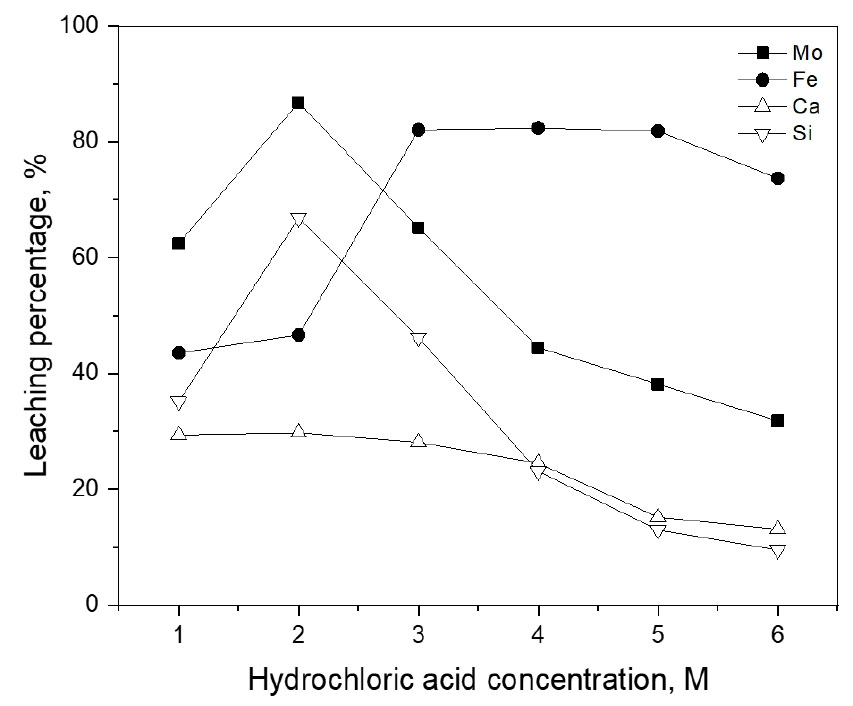
The effect of hydrochloric acid concentration was investigated by varying from 1.0 M to 6.0 M under the conditions of 0.5 M NaClO3, 20 g/L, 240 min at 70°C, and 400 rpm. Fig. 3 shows that when HCl concentration increased from 1.0 to 2.0 M, the leaching percentage of molybdenum, iron, calcium, and silicon increased from 62.5% to 86.6%, 43.5% to 46.6%, 29.4% to 29.8% and from 35.14% to 66.8%, respectively. However, when HCl concentration increased from 2.0 to 6.0 M, the leaching percentage of the metals decreased. Namely, the leaching percentage of molybdenum, iron, calcium, and silicon decreased to 31.8%, 73.7%, 13.1%, and 9.5%, when the concentration of HCl was 6.0 M. In molybdenite, iron, calcium, and silicon exist as oxides19) and their reactions in acidic solution are described by the following equations:
In a strong oxidizing solution, the dissolved metal ions would exist in the highest oxidation state. Therefore, the dissolved molybdenum in our experimental conditions would exist as Mo(VI). According to Ryzenko20), the cationic species of Mo(VI) in a strongly acidic solution (pH < 1) would be MoO22+. The leaching reaction of molybdenum can be represented as follows
The final pH of the leaching solution in this work was lower 0.32, so it can be said that molybdenite would be leached as MoO22+ in our experimental conditions.
The decrease in the leaching percentage of Mo(VI) when HCl concentration was higher than 2.0 M may be ascribed to the formation of passive film on the surface of the ores like elemental sulfur. In general, these passive films can be dissolved by a pitting mechanism in the presence of chloride ions. Therefore, more thorough experiments are needed to elucidate the phenomena in the leaching of the molybdenite ore in strong HCl solutions.
3.3. Effect of sodium chlorate concentration
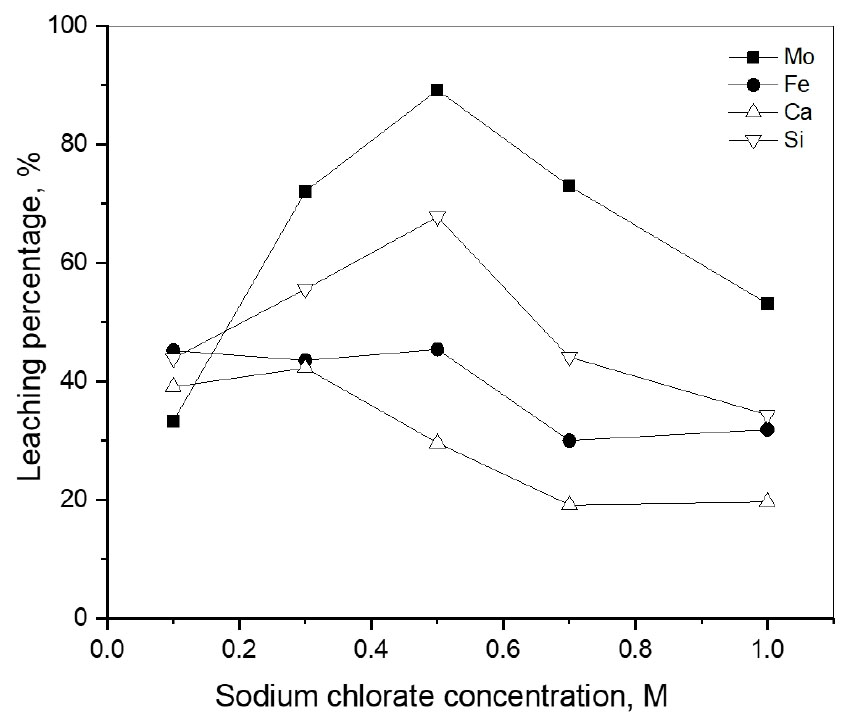
The concentration of sodium chlorate also affects the leaching of molybdenum from molybdenite ore. Therefore, the concentration of NaClO3 was varied from 0.1 M to 1.0 M under the conditions; 2.0 M HCl, pulp density 20 g/L, 240 min, 70°C, and stirring speed 400 rpm. Fig. 4 shows that when the concentration of NaClO3 increased from 0.1 M to 0.5 M the leaching percentage of molybdenum and silicon increased from 33.3% to 89.2% and from 43.9% to 67.8%, respectively. However, the leaching percentage of molybdenum and silicon decreased to 53.2%, and 34.3% as NaClO3 concentration increased further to 1.0 M. The leaching percentage of calcium increased from 39.1% to 42.3% when the concentration of NaClO3 increased from 0.1 M to 0.3 M then decreased to 19.6% at 1.0 M, while that of iron decreased from 45.2% to 31.9% at 1.0 M. Therefore, the sodium chlorate concentration of 0.5 M was chosen for further experiments. When the concentration of NaClO3 was increased from 0.1 to 0.5 M, the sulfide in molybdenite can be oxidized to sulfate. This sulfate can form precipitate with calcium ion which were leached. Actually, some precipitates were observed after the leaching experiments. The reaction can be described according to the equation:
However, the leaching percentage of molybdenum decreased when the concentration of sodium chlorate was higher than 0.5 M. It can be explained that the reduction of NaClO3 needs the participation of hydrogen ion, leading to an increase in the pH of the solution. In this case, H2MoO4 which exists in the pH range of 2.5 - 6.5 may form21). CaSO4·2H2O can encapsulate or adsorb H2MoO4, resulting in the relatively low leaching of molybdenum22). The reaction can be described according to the equations:
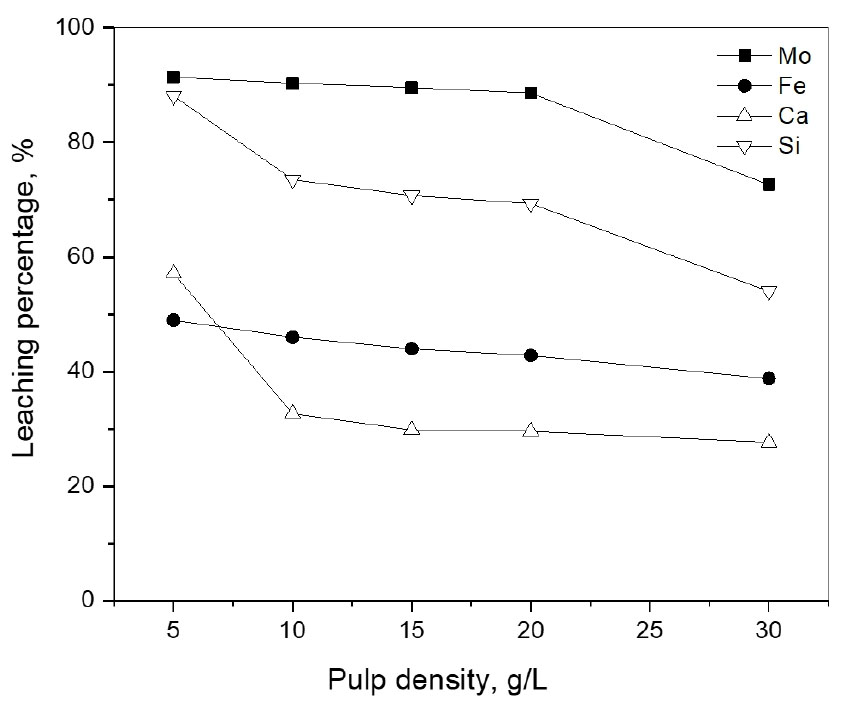
3.4. Effect of pulp density
The effect of pulp density was investigated between 5 g/L and 30 g/L under the conditions of 2.0 M HCl, 0.5 M NaClO3, 240 min, 70°C, and 400 rpm, and the results are presented in Fig. 5. As can be seen, the leaching percentage of molybdenum, silicon, iron, and calcium decreased significantly from 91.3% to 72.6%, 88.1% to 53.9%, 48.9% to 38.7%, and 57.1% to 27.7%, respectively, with increasing pulp density from 5 to 30 g/L. When pulp density increases, the amount of leaching solution and the oxidizing agent would be deficient, and thus the leaching percentage of the metals decreases. Therefore, 5 g/L pulp density was chosen as the optimum for the leaching of the ores.
3.5. Effect of reaction time
In order to investigate the effect of reaction time, leaching experiments were performed by varying reaction times from 30 to 300 mins under the following conditions; 2.0 M HCl, 0.5M NaClO3, 5 g/L, 70°C, and 400 rpm. Fig. 6 shows that the leaching percentage of Mo(VI) increased from 74.8% to 89.5% with an increased reaction time from 30 to 60 min. Similarly, The leaching percentage of iron, calcium, and silicon also increased from 28.8% to 54.9%, from 17.3% to 40.3%, and from 28.4% to 79.8%, respectively. Our data indicate that further experiments are needed to investigate the leaching kinetics of molybdenite by HCl solution containing NaClO3.
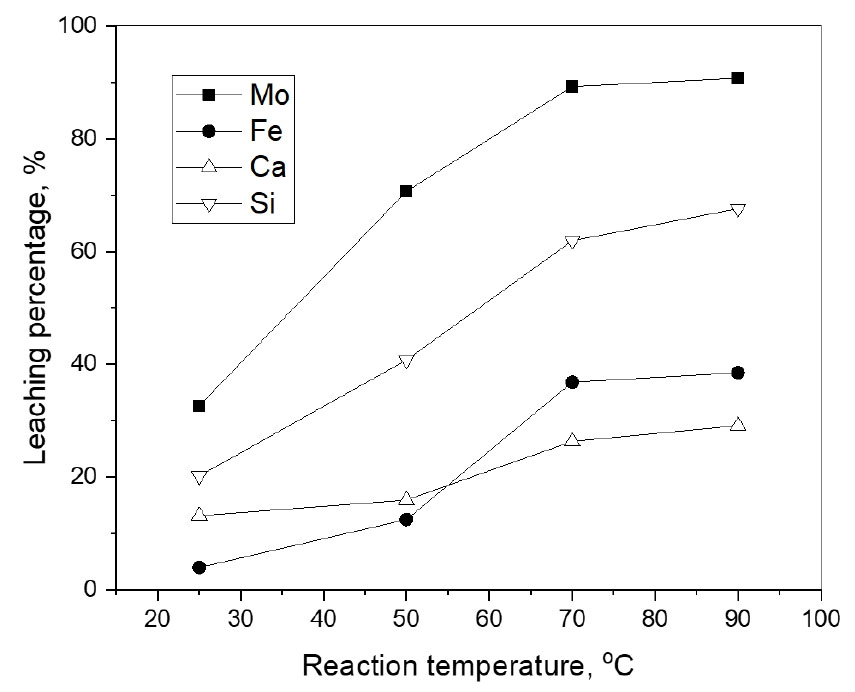
3.6. Effect of temperature
In order to investigate the effect of temperature, leaching experiments were performed by varying reaction temperature from 25°C to 90°C under the following conditions; 60 min, pulp density 5 g/L, 2.0 M HCl, 0.5 M NaClO3, and 400 rpm. Fig. 7 shows that the leaching percentage of molybdenum and silicon increased from 32.6% to 90.7% and from 20.2% to 67.6%, respectively when the reaction temperature increased from 25 to 90°C. In this temperature range, the dissolution percentage of calcium and iron also increased from 13.1% to 29.1% and from 3.9% to 38.5%, respectively. The increase in the leaching percentage of the metals with reaction temperature might suppose that the leaching of the molybdenite by HCl and NaClO3 is controlled by the chemical leaching step. However, more kinetic data is to be obtained to verify the rate-determining step of the leaching of the molybdenite ore. From the obtained data, an optimum leaching condition of molybdenite ore by the mixture of HCl and NaClO3 was as follows; 2.0 M HCl, 0.5 M NaClO3, 5 g/L pulp density, 90°C, and 60 min. The leaching percentage of molybdenum, iron, calcium, and silicon at this condition was 90.7%, 38.5%, 29.1%, and 67.6%, respectively.
4. Conclusions
Oxidative leaching of domestic molybdenite ore was investigated by using hydrochloric acid as a lixiviant. Among the three kinds of oxidizing agents employed in this work (NaClO3, NaClO, and H2O2), NaClO3 was the most effective in leaching molybdenum from the ore. The effect of some variables such as the concentration of HCl and NaClO3, pulp density, reaction time, and temperature was investigated. Since the ores contained CaO, the dissolved Ca(II) can form precipitates with sulfate ions resulting from the oxidation of the sulfide in the ore. Therefore, the precipitation of gypsum and sulfur layer on the surface of the reacting ores might be responsible for the decrease of leaching percentage in strongly acidic and oxidizing conditions. Among the several variables, reaction temperature affected the leaching percentage of molybdenum from the ore. Under the optimum conditions (2.0 M HCl, 0.5 M NaClO3, pulp density of 5 g/L, 90°C for 60 min, and 400 rpm), the leaching percentage of molybdenum was greater than 90%, while that of iron, calcium, and silicon was 38.5%, 29.1%, and 67.6% respectively.