1. Introduction
2. Experimental
2.1. Materials, reagents apparatus
2.2. Liquid-liquid extraction process
3. Results and discussions
3.1. Liquid-liquid extraction and separation of U/V with Alamine 336
3.2. Liquid-liquid extraction and separation of U(VI)/ V(V) by Alamine 308
3.3. Solvent extraction and separation of U and V by Alamine 304
3.4. Solvent extraction and separation of U(VI)/ V(V) by Aliquat 336
4. Conclusions
1. Introduction
Energy requirements are changing very fast in the new millennium, renewable energy and nuclear energy demand is increasing. Currently, the people’s daily life depend on the electrical and electronic goods and the economic growth of the country mainly depends on the exports and imports of goods to other countries. The industrial development and cultivation depends on the electricity of developing/undeveloped countries.
Uranium is the key source of nuclear power generation as an economic resource while the increase in the electricity availability would help fulfilling the country’s demands. After Second World War, all over the globe the radioactive elements demand grew vigorously. in order to protect the environment as well as recycling the precious/ rare metals, the researchers focus their aims to separate the rare metals and remove the toxic species through eco-friendly methods. Metallurgy deals the pyro- and hydrometallurgical branches. Specifically, in hydrometallurgy there is the possibility to develop eco-friendly techniques, which have attracted the researchers worldwide towards this field.
Various amine and amide based extractants have been employed in the uranium recovery and possible separation from other associated elements at different acidic media1,2,3,4,5,6,7,8,9,10,11,12).
The latest available studies show very limited attempts for the vanadium extraction by amine-based extractants13,14,15,16,17,18); in addition, there is only a few reported research papers available for the separation technology between uranium and vanadium.
The extraction behavior of new bi-functional ligands containing sulfur and amide groups towards the U(VI) ion from nitric acid was studied and reported19,20,21,22,23,24). Other reports are related to P based extractants used for U extraction25,26,27,28,29) and our previous extraction studies on U extraction was developed and reported30,31,32,33).
Extraction and possible separation of U from its associated elements, vanadium, is imperative bearing in mind the rising demand for nuclear power applications. In this work, a simple process is considered to separate uranium from vanadium by its extraction. The present title metals concentrations were prepared based on the yellow cake leach liquor proportions. The yellow cake leach liquor contains uranium leached along other elements predominantly vanadium. The main target and principal objective of the present study is as much as possible separate the vanadium from low-grade uranium ore leach liquors. This is the first report on extraction and separation of U(VI) and V(V) by amine based extractants in sulfuric acid solutions.
2. Experimental
2.1. Materials, reagents apparatus
Analysis of U(VI) and V(V) content in aqueous solutions phase is obtained using ICP-OES Perkin Elmer Model Optima 2000 Dr. The amine-based extractants (supplied by Cognis Corporation USA) information is described in Table 1 and were used as it is without purification (Table 1). Usually, the extraction using amine- based extractants follows a two-step reaction, protonation and ion exchange:
Where [R3N] denotes Alamine 336, 308 or 304
During the research kerosene, supplied by Junsei Chemicals Co. Ltd., Japan, was mixed for dilution, and all other reagents used were analytical grade.
Table 1.
Chemical and physical properties of extractants
2.2. Liquid-liquid extraction process
Two immiscible liquids (Aqueous phase (A) / Organic phase (O), each phase 30 mL), an aqueous phase containing the title metals and an organic phase having the extractant mixed for dilution in kerosene, are contacted inside a mechanical shaker using separating funnels at the optimized time and temperature (25±0.5°C). After extraction and phase disengagement, the aqueous phase is analyzed to obtain the metal content by ICP-OES, using mass balance the metal concentration in the organic phase can be calculated30,34,35).
The preliminary experiments were tested on time influence on both title metals it indicates that within 15 min reaching the extraction equilibrium. The current research for uranium(VI) and vanadium(V) separation using amine-based extractants followed the constant experimental conditions: time = 30 min, aqueous : organic (A:O) = 1 (unit phase ratio) and temperature = 25°C.
3. Results and discussions
3.1. Liquid-liquid extraction and separation of U/V with Alamine 336
3.1.1. Extraction
3.1.1.1. Sulfuric acid effect
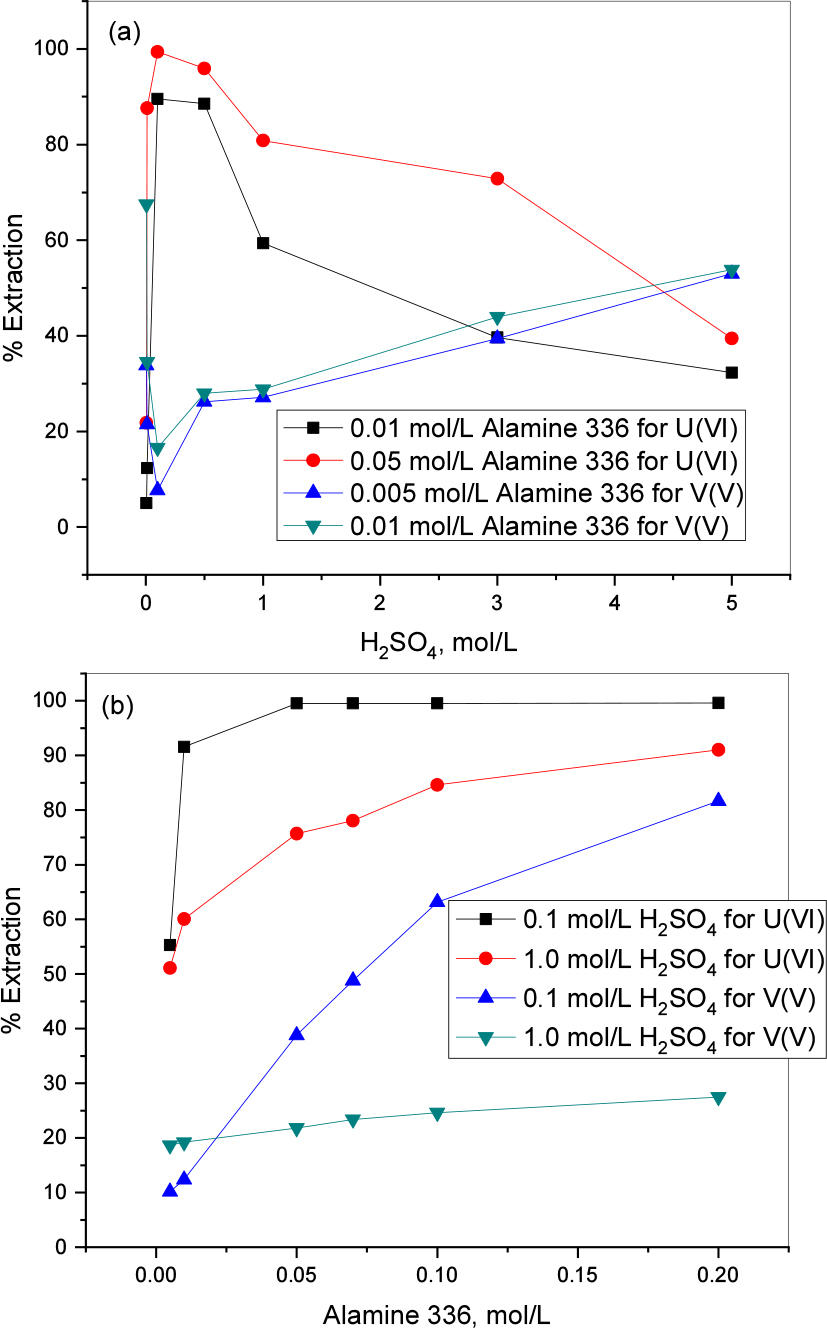
The preliminary experiment was conducted to know the extraction behavior of uranium and vanadium in sulfuric acid media (range: 0.005 to 5.0 mol/L H2SO4)36). For experiments, the concentration of the metals was kept constant at1 mmol/L uranium(VI) and 4 mmol/L vanadium(V). While, the concentration of extractant was modified and various concentrations of extractant like 0.005, 0.01, 0.05 mol/L Alamine 336. The extraction behavior of the uranium shows positive influence up to 0.5 mol/L of acid concentration later on it gradually decreases with increasing acid concentration up to 5.0 mol/L. In the case of vanadium, the extraction behavior shows an opposite trend when compared with uranium extraction phenomena. That means up to 0.1 mol/L acid concentration the percentage extraction decreased with the rise of the acid concentration while it slowly rises and reaches below ~54% in both cases of extractant concentration (Fig. 1(a)).
3.1.1.2. Extractant effect
Influence of extractant (Alamine 336) on the extraction behavior of 1 mmol/L uranium(VI) and 4 mmol/L vanadium (V) was tested in the range of 0.005 to 0.2 mol/L Alamine 336 at 0.1 and 1.0 mol/L sulfuric acid concentrations. The extraction efficiency increased with the extractant concentration augmentation in both acidic conditions. Fig. 1(b) shows the results of the experiment and it indicates that both metals show good extraction ability with Alamine 336 at 0.1 mol/L H2SO4 concentration. This result determines that lower acidic conditions are favorable to uranium and vanadium extraction.
3.1.2. Separation
In the field of separation science, separation factor (SF) plays a key role. To calculate the SF by following equation:
Separation factor (SF) β = DU(VI) / DV(V)
Where β = separation factor; DU(VI) = distribution ratio of the uranium; DV(V) = distribution ratio of the vanadium.
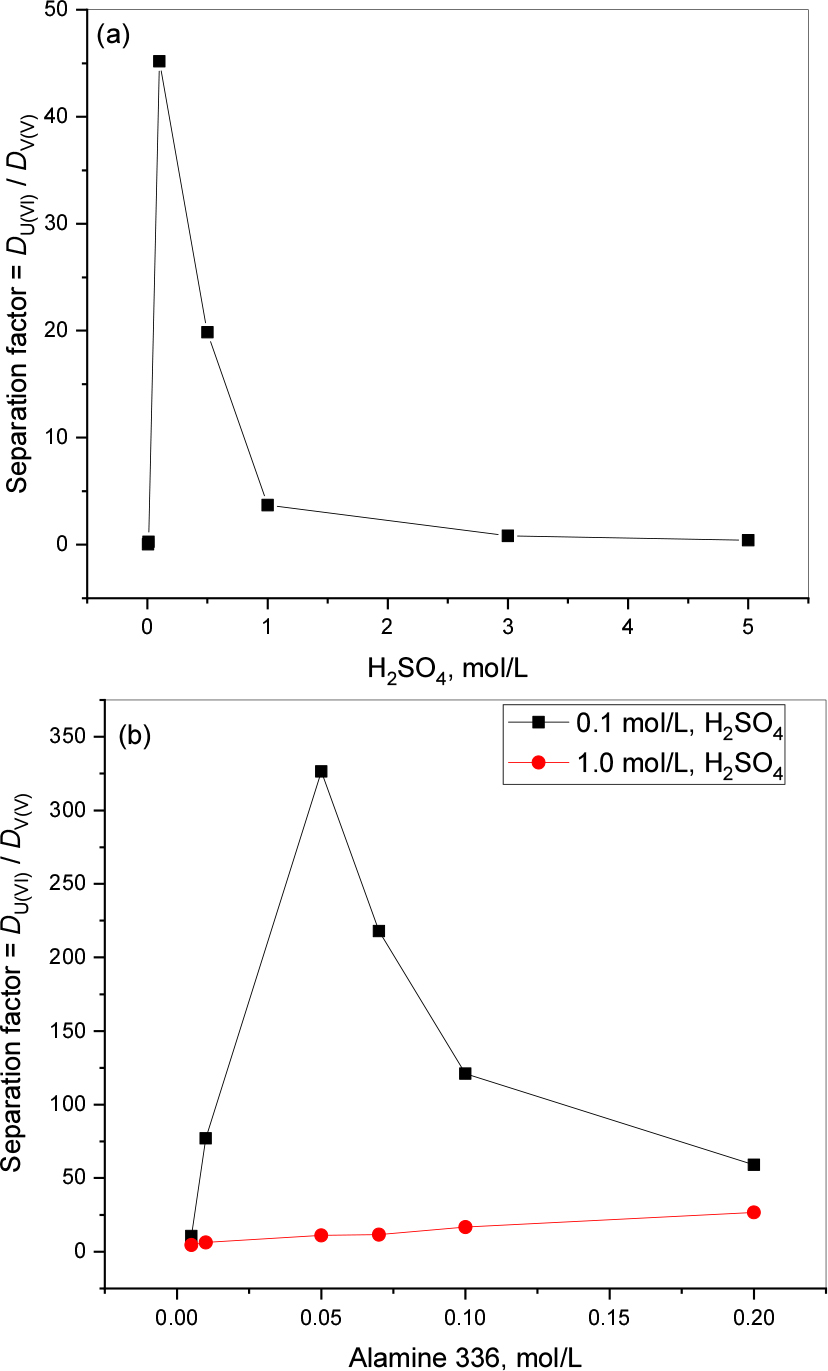
From the sulfuric acid and extractant effect data we calculated the SF’s for uranium and vanadium and presented as Figs. 2(a) and (b). Fig. 2(a) represents the acid effect data, it shows that 0.1 mol/L is much favorable separation condition (SF = 45.18) for uranium and vanadium when compared to other conditions. Similarly, we presented the extractant effect data as Fig. 2(b). At 0.1 mol/L acid concentration there is the highest SF (326.49) with 0.05 mol/L whereas at 1.0 mol/L H2SO4 it requires a higher extractant concentration (0.2 mol/L Alamine 336) even though the SF is 26.72, it is a very low value when compared with the lower acidic condition SF. The present results determine that lower acidic conditions is better for uranium and vanadium separation.
3.2. Liquid-liquid extraction and separation of U(VI)/ V(V) by Alamine 308
3.2.1. Extraction studies
3.2.1.1. Sulfuric acid effect
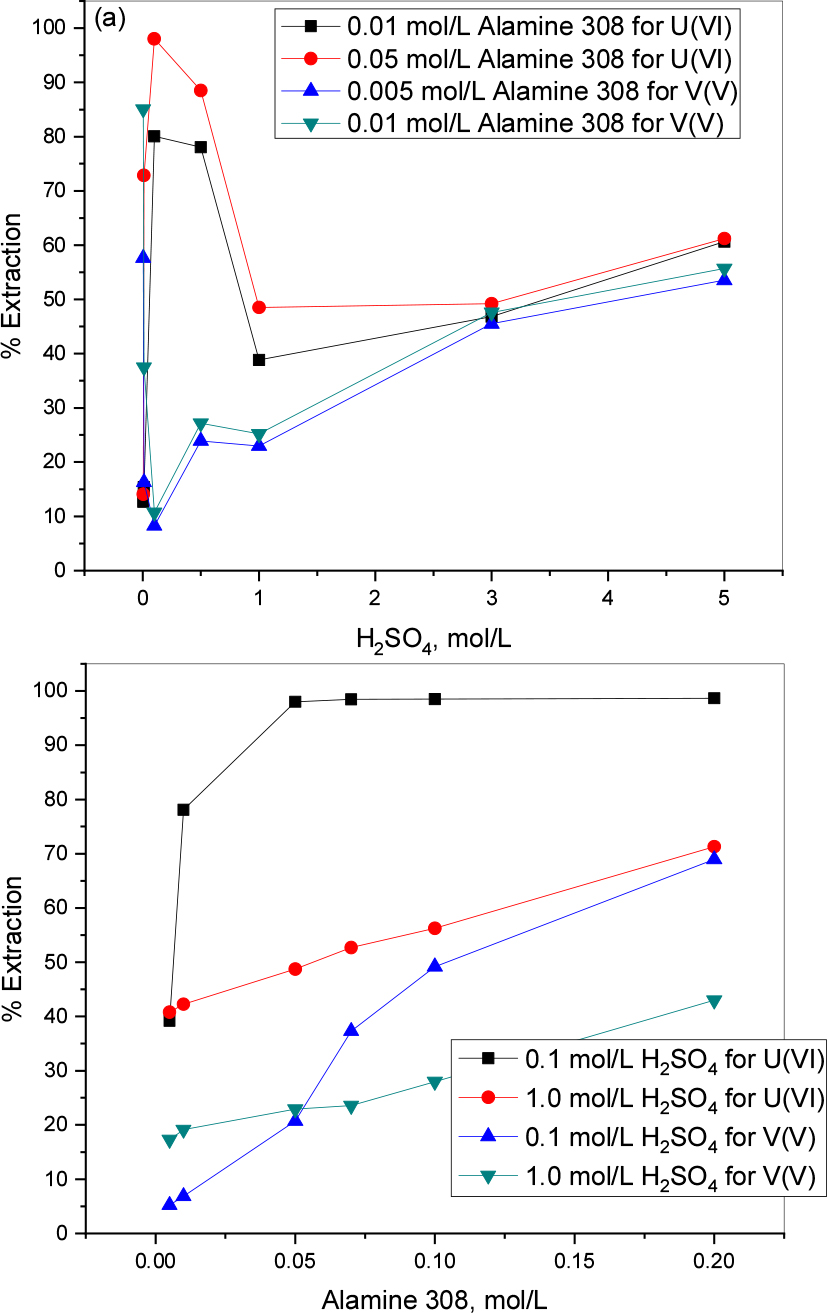
Fig. 2(a) represents the sulfuric acid effect on U(VI) and V(V) extraction using Alamine 308. The extraction has a greater positive influence in lower acidic conditions and it reaches ~98% at 0.1 mol/L H2SO4 concentration with 0.05 mol/L Alamine 308; even with 0.01 mol/L extractant it shows an ~80% uranium extraction. After that, the curve decreases with the increase of acid concentration up to 3.0 mol/L (H2SO4 concentration); whereas at higher acidic conditions like above 5.0 mol/L H2SO4 the percentage of uranium extraction slightly increases. In the case of vanadium(V), increasing the acidity makes the extraction percentage decrease up to 0.1 mol/L, showing an opposite trend to the uranium extraction. Then, the percentage extraction starts increasing with acidity reaching higher levels like 5.0 mol/L H2SO4 and it shows good results for separation in between both metals.
3.2.1.2. Extractant effect
Alamine 308 is a water insoluble, tri-iso-octyl amine usually used to selectively extract cobalt in chloride leach liquors and sometimes for tungsten extraction. In the present study we proposed Alamine 308 as extractant for uranium(VI) and vanadium(V) extraction as well as separation. The experimental results are presented in Fig. 3(b) and show a progressive increasing extraction behavior with the increase of the extractant concentration in both acidic conditions. It clearly indicates that, lower acidic conditions are more favorable for uranium and the maximum extraction is ~98.7% of uranium and ~69% vanadium at 0.1 mol/L acidic condition with 0.2 mol/L extractant concentration.
3.2.2. Separation possibilities
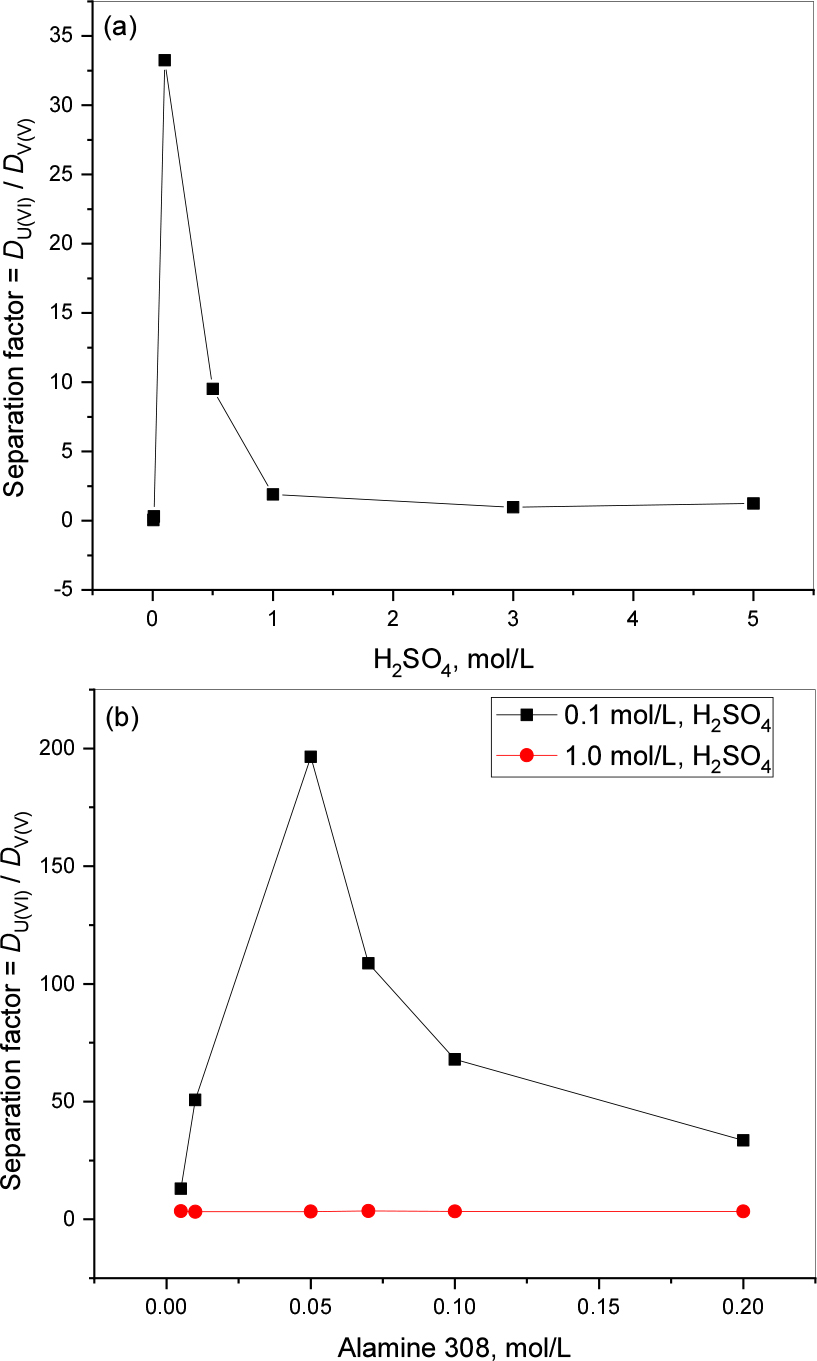
The results of separation of U(VI) and V(V) with 0.1 mol/L Alamine 308 was presented in Fig. 4(a) and (b). Fig. 4(a) showing the SF when acid effect was studied, the highest SF is 33.25 at 0.1 mol/L acid concentration. Fig. 4(b) showing the highest SF (196.48) with 0.05 mol/L extractant concentration at 0.1 mol/L acidity and whereas at1.0 mol/L acidity SF are very low when compared with lower acidity (3.4±0.3).
3.3. Solvent extraction and separation of U and V by Alamine 304
3.3.1. Extraction studies
3.3.1.1. Sulfuric acid effect
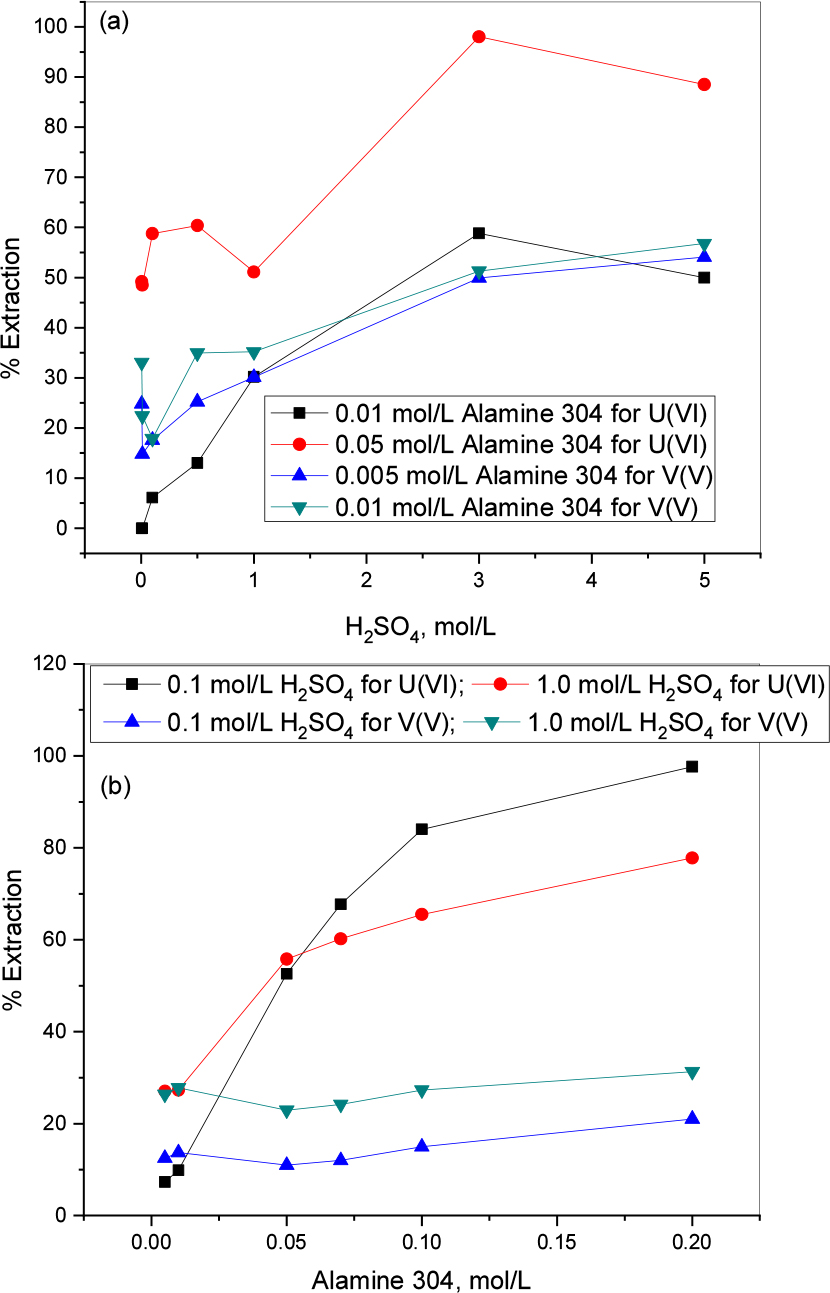
The percentage extraction of U(VI) and V(V) from sulfuric acid (0.005 to 5.0 mol/L range) solution with Alamine 304 diluted in kerosene as extractant is presented in Fig. 5(a). It indicates that with 0.01 mol/L Alamine 304 at lower acidic levels (0.005 and 0.01 mol/L H2SO4) there is no significant extraction of uranium while at 3.0 mol/L acidity it reaches ~59% extraction. At the same acidity 98% of uranium is extracted by 0.05 mol/L Alamine 304. In the case of vanadium(V), ~57% is possible to extract with 0.01 mol/L Alamine 304 at 5.0 mol/L H2SO4.
3.3.1.2. Extractant effect
Extractant effect (Alamine 304) was tested using the constant concentrations of 1 mmol/L uranium and 4 mmol/L vanadium in 0.1 and 1.0 mol/L sulfuric acid solutions. ~98% of uranium (VI) and 21% of vanadium (V) were extracted at 0.1 mol/L H2SO4 with 0.2 mol/L Alamine 304. A 1.0 mol/L acidity is favorable for vanadium extraction (Fig. 5(b)), while 0.1 mol/L acidity is more favorable for uranium extraction.
3.3.2. Separation possibilities
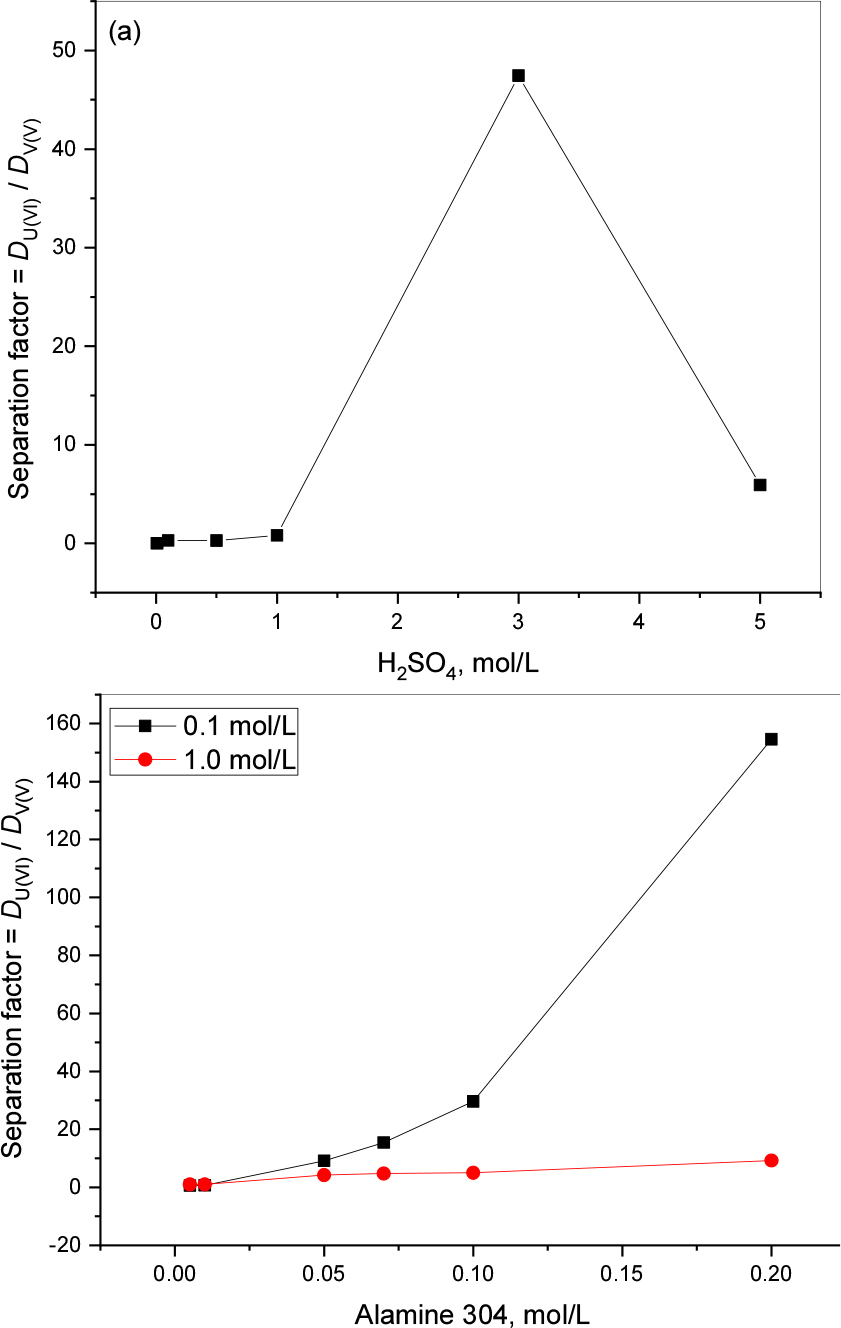
Separation factors for uranium and vanadium with Alamine 304 were calculated for acid and extractant effect and presented in Fig. 6(a) and (b). Fig. 6 (a) showing highest SF (47.44) observed at 3.0 mol/L H2SO4 while at other acidic conditions separation is not efficient. The trend of the SF is increasing with increasing of the extractant concentration for both title metals. As per Fig. 6(b) data 0.2 mol/L of Alamine 304 shows the highest SF = 154.58.
3.4. Solvent extraction and separation of U(VI)/ V(V) by Aliquat 336
3.4.1. Extraction studies
3.4.1.1. Sulfuric acid effect
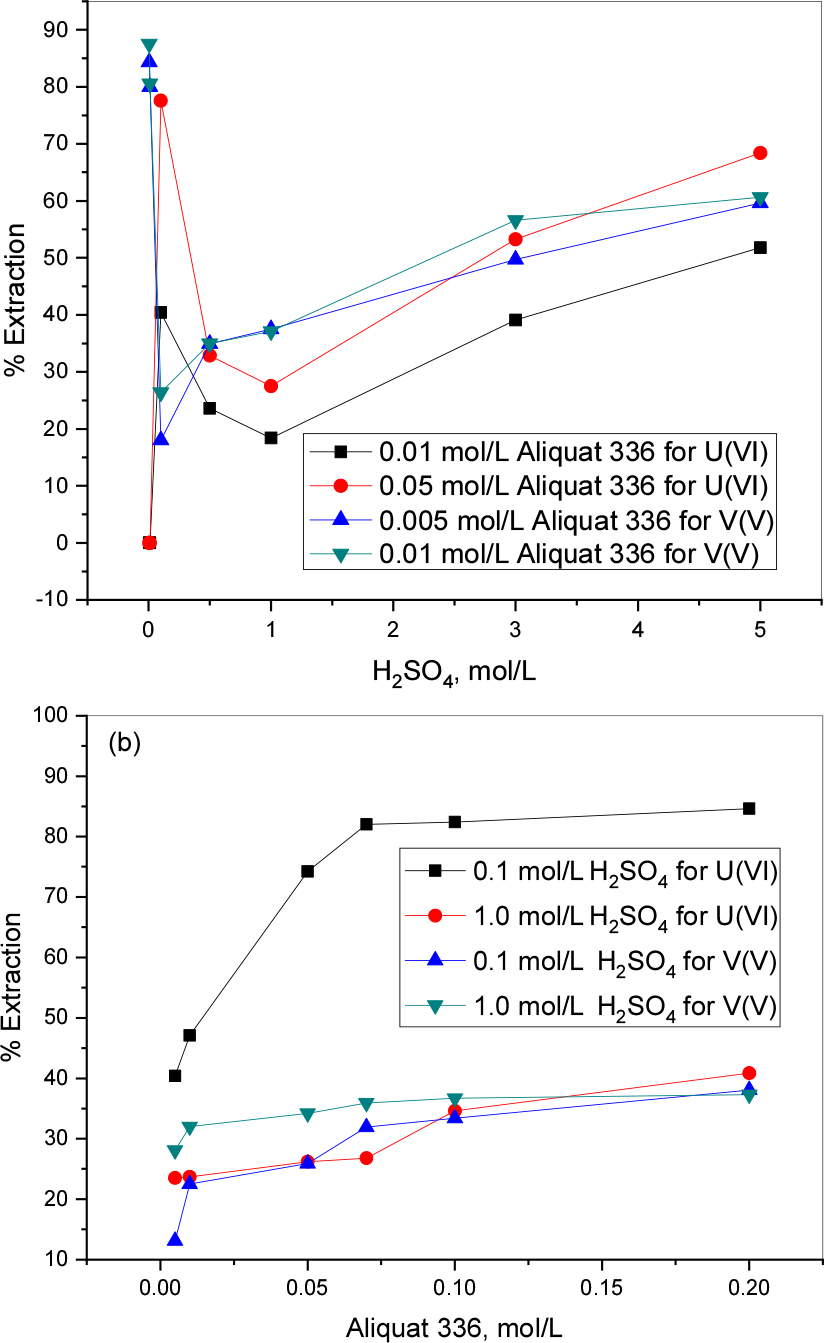
Extraction behavior of 1 mmol/L uranium(VI) at lower acidity levels (0.005 and 0.01 mol/L H2SO4) is minor with 0.01 and 0.05 mol/L Aliquat 336. The trend of extraction shows that until 1.0 mol/L H2SO4 the extraction decreases with increasing acidity and at higher levels (3.0 and 5.0 mol/L H2SO4) it reached ~52 % for 0.01 mol/L Aliquat 336 and ~68 % for 0.05 mol/L Aliquat 336 of uranium. In the case of V (V) lower levels of acidity (0.005 and 0.01 mol/L H2SO4) are favorable for extraction and it reaches ~88% with 0.01 mol/L Aliquat 336 while the percentage of extraction decreased with the increase in acid concentration (Fig. 7(a)).
3.4.1.2. Extractant effect
Aliquat 336 is a quaternary ammonium salt, water insoluble and made by the methylation of mixed tri- octyl/decyl amine. The current research we tested the uranium(VI) and vanadium(V) possible separation. Results show that the extracting ability will increase with the increasing of extractant concentration and reaches ~85% of uranium and ~38% of vanadium extraction by 0.2 mol/L Aliquat 336 at 0.1 mol/L H2SO4.
3.4.2. Separation possibilities
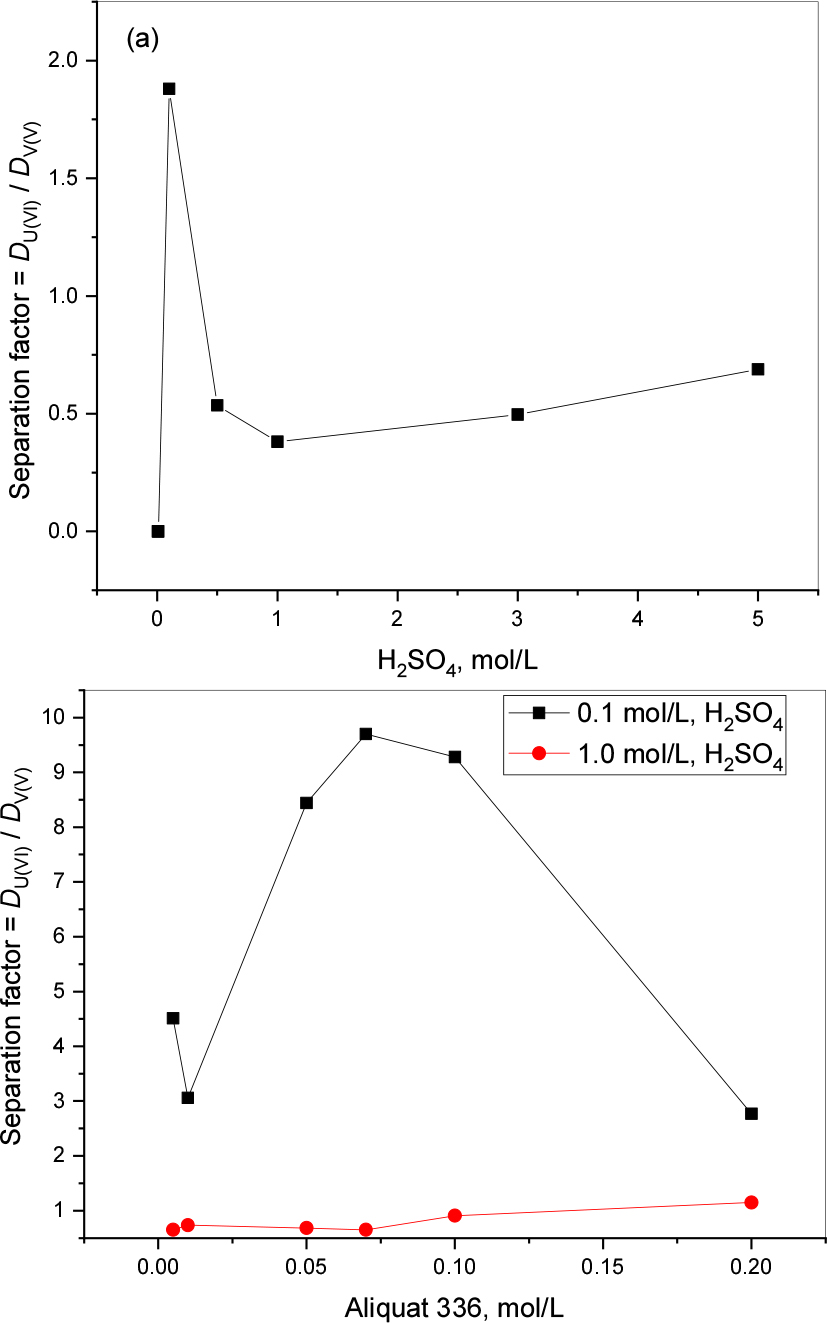
Calculated separation factors are presented in Fig. 8 (a) and (b) for acid and extractant concentration effects. Results indicate a very low SF in both cases (1.88 and 9.7). These SF are minor compared with the other amine- based extractants studied.
Considering the discussed experimental results, four amine-based extractants such as Alamine 336, 308,304 and Aliquat 336 were used for uranium and vanadium extraction and separation giving following summary of the results on separation factors (Table 2). The lower sulfuric acid concentrations (0.005 to 0.5 mol/L H2SO4) are much favorable for extraction as well as separation of uranium and vanadium. Alamine 308 extracted 80 and 98 % of uranium at lower acidity (0.1 mol/L) using 0.01 and 0.05 mol/L extractant concentrations. Whereas in the case of Alamine 304 as extracting agent, at reduced acidic levels the extraction of uranium is negligible while ~57% of vanadium was extracted. Similar behavior was noted in the case of Aliquat 336 as an extractant, lower levels of acidity are not favorable for uranium extraction but favorable for vanadium (~88% extracted). The summary of the experiments led to the conclusion that the optimal separation of uranium and vanadium was possible at lower sulfuric acidic conditions (0.1 mol/L) with 0.05 mol/L Alamine 336 as an extractant.
Table 2.
Comparison of separation factors (β = DU(VI) / DV(V)) of uranium and vanadium
4. Conclusions
The current experimental research was developed for uranium(VI) and vanadium(V) possible separation and extraction from yellow cake leach liquors in sulfuric acid media. Four amine- based extractants, Alamine 336, 308, 304 and Aliquat 336 were investigated as potential extractants for the U(VI) and V(V) separation process from sulfuric acid leach solutions. Available literature demonstrates that very limited number of technologies were studied for the separation of uranium and vanadium. From the present experimental results, the following conclusions are drawn:
1. Highest SF was obtained with 0.05 mol/L Alamine 336 as extractant (SF = 326.49) in 0.1 mol/L H2SO4 media
2. With 0.05 mol/L Alamine 308 it reaches SF = 196.48, 0.2 mol/L Alamine 304 SF = 154.58 and 0.07 mol/L Aliquat 336 SF = 9.28 at 0.1 mol/L H2SO4 concentration
3. The present investigation clearly determines that Alamine 336 is the optimum reagent for separation of uranium and vanadium even at lower concentrations of extractant (0.05 mol/L) when compared with other amine-based extractants
4. Finally, the order of the SF is as follows: Alamine 336 > Alamine 308 > Alamine 304 Aliquat 336