1. Introduction
2. Experimental
2.1. Materials
2.2. Solvent extraction and stripping procedure
3. Results and discussions
3.1. Effect of acid and extractant concentrations
3.2. Effect of metal concentrations in the feed solution on the metal extraction
3.3. Effect of phase ratio on the extraction of metals
3.4. Stripping of Ag(I) from the loaded LIX63
3.5. Effect of phase ratio on the stripping of Ag(I) with ammonia water
3.6. Process flowsheet for the separation of Ag(I) and Al(III) with LIX63
4. Conclusions
1. Introduction
Silver has been widely used in aesthetic, industrial, and technical applications1,2,3,4). In manufacturing a photovoltaic module, silver (Ag) is used as front side metallization of silicon solar cells5). The rapid increase in the production and installation of photovoltaic systems has increased the importance of recycling photovoltaic modules. However, most of the recycling research efforts in the photovoltaic module recycling field have focused on silicon (dominant composition of solar cells). Some researches included recycling of glass and/or aluminum. The removal of silver was taken as part of the steps to prepare the silicon wafer to be reused. Some studies did not even include silver recovery5).
The growing demand for silver and the increasing strictness of environmental policies have made silver recovery from various secondary resources an attractive proposition5,6,7). The mainly used method in Ag(I) recovery/removal from photovoltaic modules (silicon solar cells) is etching in acidic solutions based on nitric acid7,8,9,10,11). Table 1 summarizes some studies on Ag(I) recovery from photovoltaic modules. Based on the literature, Ag(I) can be recovered from various nitrate solutions (mainly from X-ray film and photographic waste) by electrolysis, chemical precipitation12), solvent extraction13), adsorption2,14) and cementation15), among others. Solvent extraction is a potential technique for recovering metal ions from solutions because of its important advantages, such as simplicity, flexibility, economic benefits, rapidity, lower costs, and being environmentally friendly, among others12,16).
Table 1.
Reported studies on silver recovery from solar modules
| Target metal | Method | Reference |
| Ag |
Leaching with 64% HNO3, then precipitation with NaCl (the Ag concentration yield is 94%) Subjected to pyrolysis at 500 °C before the same leaching process (the Ag concentration yield is 92%) | 7) |
| Si |
Leaching with 40% HNO3 at 40 °C for Ag removal Leaching with 30% KOH at 80 °C for Al removal | 8) |
| Si |
Leaching with 30% KOH for Al removal Leaching with mixture of 40% HF, 65% HNO3, and 99.5% CH3COOH and Br2 for Ag coating, AR coating, and n-p junction removal | 9) |
|
Glass Si |
Tempered glass is recovered using organic solvents Immersion in the etching solution combined with surfactants for removal of coatings (Ag, Al together with others), n-p junction 99.99% pure Si is obtained | 10) |
|
Ag Cu Al |
Metals (Ag, Al, Cu, Pd) were dissolved by 5 mol/L HNO3 at 200 rpm Al is precipitated with KOH Cu is recovered by extraction with LIX84I followed by stripping with H2SO4 Ag is recovered by reduction with hydrazine hydrate (purity: 99.7%) | 11) |
The chelating agent, 5,8-diethyl-7-hydroxydodecan-6-oxime (LIX63), was reported to have a selectivity on silver from nitrate solutions containing Ag, Zn; or Ag, Ca, Fe, Si, Re and Zn17,18). Sodium thiosulfate and high concentration of HNO3 was used for stripping of Ag from the loaded LIX6317,18). In this study, in order to separate and recover Ag and Al from the nitrate leach solution of a solar cell, LIX63 was employed as extractant to extract Ag(I) while ammonia water was used as a strippant. The leach solution which was obtained in our previous study contained 0.01 mol/L Ag, 0.195 mol/ L Al and 0.4 mol/L HNO3. Several parameters, such as, metal concentration in the solution, concentration of the strippant, volume ratio of aqueous to organic phases on the extraction/stripping efficiency, were investigated. The feasibility of the recovery process was verified by running batch simulation of counter-current extraction and stripping.
2. Experimental
2.1. Materials
A synthetic leach solution was used in the extraction experiments because of the limited amount of the real leaching solution. The feed solution was prepared by dissolving the necessary amounts of AgNO3 (99.8%, Daejung Chemicals and Metals Co., Ltd.) and Al(NO3)3·9H2O (98.0%, Daejung Chemical and Metals Co., Ltd.) in distilled water. The Ag(I) and Al(III) concentrations in all the experiments were maintained at 0.01 and 0.195 mol/L, respectively, to mimic the leach solution. HNO3 (60%, Daejung Chemical and Metals Co., Ltd.) was used to adjust the acidity of the solution.
The extractant (i.e., LIX63) was purchased from Cognis and used without further purification. Kerosene (90% distillation under 265℃, Daejung Chemicals and Metals Co., Ltd.) was used as the diluent, while ammonia water (25%, Junsei Chemical Co., Ltd.) was used in preparing the strippant solution. The strippant solution was prepared by dissolving the required amount of reagents in the desired volume of distilled water. All reagents used were of analytical grade.
2.2. Solvent extraction and stripping procedure
General batch extraction and stripping experiments were performed by contacting an equal volume (20 mL) of the aqueous and organic phases for 30 min using a wrist shaker (Burrell, Model 75, USA). All the experiments were performed at room temperature (25 ± 1 °C). The two phases were separated using separation funnels after shaking. Subsequently, the metal ion concentration in the aqueous phase was measured via inductively coupled plasma optical emission spectrometry (ICP-OES; PerkinElmer Optima 8300). The metal ion concentration in the organic phase was determined by mass balance.
3. Results and discussions
3.1. Effect of acid and extractant concentrations
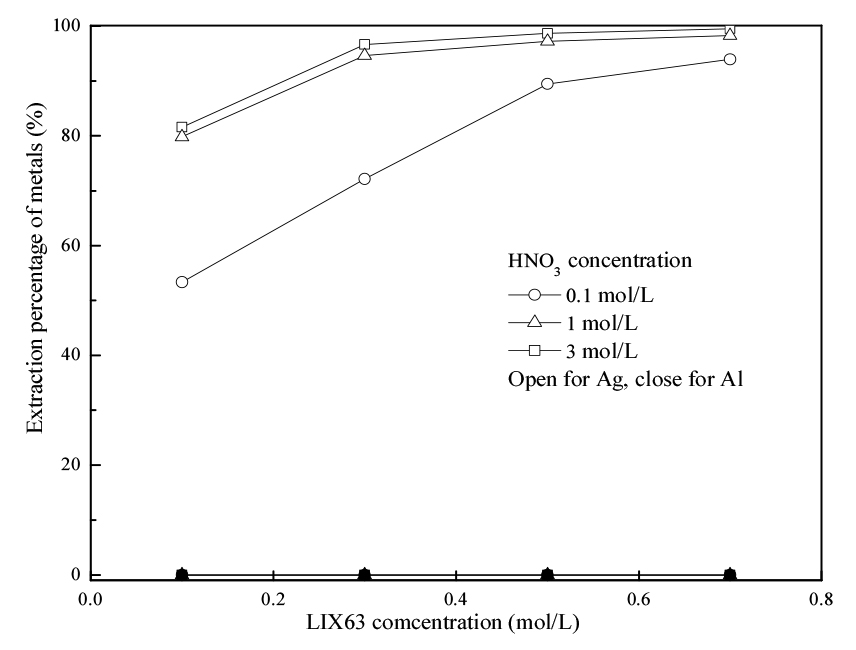
Effects of concentration of acid in the leaching solution and extractant on the extraction of Ag(I) and Al(III) were investigated by varying the concentration of HNO3 from 0.1 to 3 mol/L in the feed solution and LIX63 in the organic phase from 0.1 to 0.7 mol/L. The Ag(I) and Al(III) concentrations in these experiments were fixed at 0.01 and 0.195 mol/L, respectively. Fig. 1 exhibits the effect of the HNO3 and LIX63 concentrations on the extraction percentage of Ag(I). The extraction percentage of Al(III) in all these extraction experiments was ~0%. The extraction percentage of Ag(I) increased with the increasing LIX63 concentration at a fixed acid concentration. When the acid concentration increased from 0.1 to 1 mol/L, the extraction percentage of Ag(I) increased, while the extraction percentage was little changed (difference <5%) with further increase the acid concentration from 1 to 3 mol/L. The obtained results agree well with our previous study18). A solvating extraction mechanism responded to the extraction of Ag(I) with LIX63 when the HNO3 concentration was higher than 0.1 mol/L18). The reaction equation can be represented as follows18).
Where H2R denotes LIX63.
The results obtained in Fig. 1 indicated that LIX63 had selectivity for the extraction of Ag(I) over Al(III). Therefore, the separation of Ag(I) and Al(III) from the HNO3 solutions using LIX63 is possible.
3.2. Effect of metal concentrations in the feed solution on the metal extraction
The effect of the Ag(I) and Al(III) concentrations in the feed solution was investigated by varying the Ag(I) concentration from 0.001 to 0.01 mol/L. The molar ratio of Ag(I) to Al(III) was fixed at 1:19.5. The HNO3 concentration in the feed solution and LIX63 was fixed at 0.4 and 0.5 mol/L, respectively.
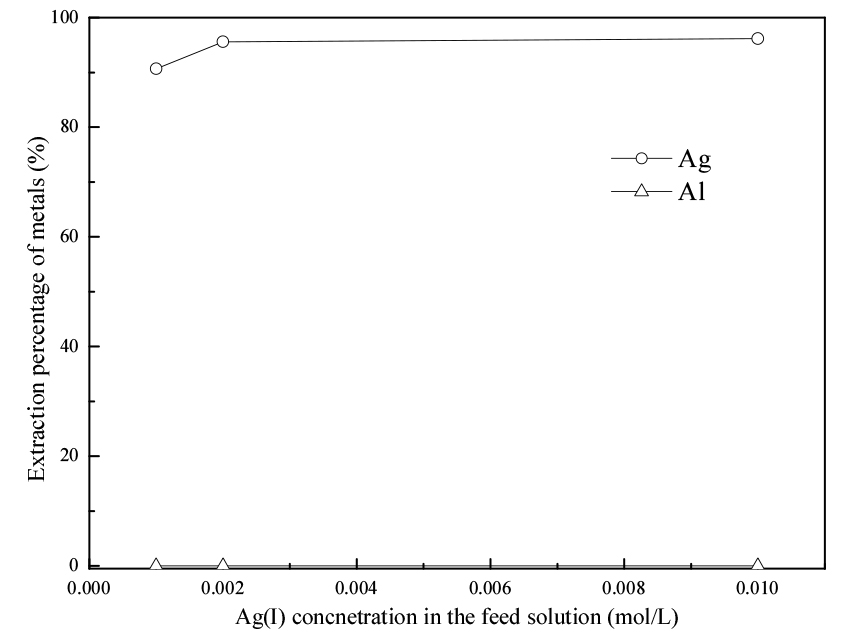
Fig. 2 shows that the extraction percentage of Ag(I) slightly increased when the Ag(I) concentration in the feed solution increased from 0.001 to 0.002 mol/L. Furthermore, the extraction percentage of Ag(I) was higher than 90% in all the studied concentration ranges.
No variation was observed in Al(III), and the extraction percentage was negligible Thus, the variation in metal concentration in the feed solution had a slight effect on the extraction of Ag(I) and no effect on the extraction of Al(III).
Based on the extraction behavior, Ag(I) and Al(III) can be separated by extraction with LIX63.
3.3. Effect of phase ratio on the extraction of metals
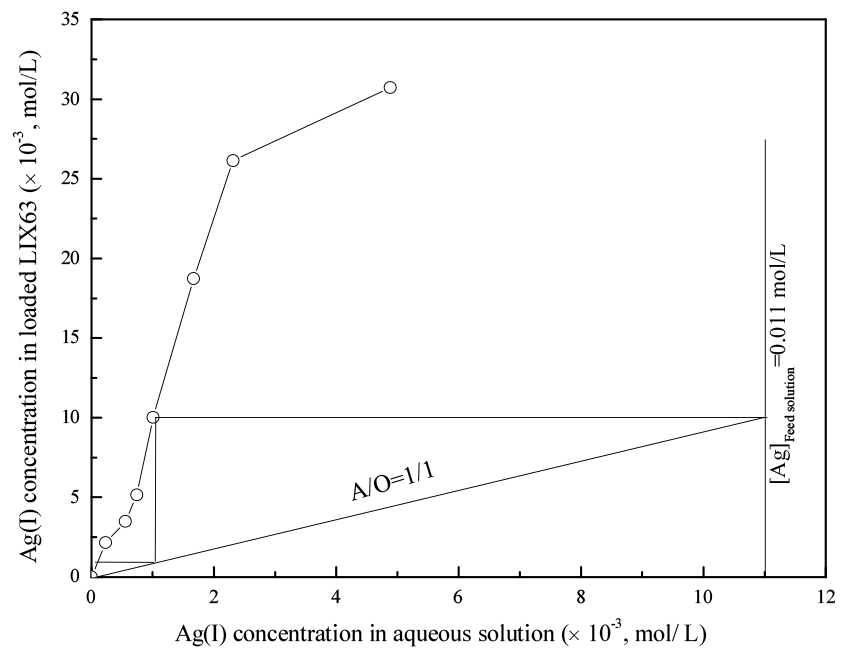
In order to determine the theoretical number of stages required for the complete extraction of Ag(I) from the synthetic leach solution containing Ag(I) and Al(III), a McCabe–Thiele plot was constructed. The LIX63 concentration in these experiments was kept at 0.3 mol/L.
The results showed that the extraction of Ag(I) increased from 55.71 to 97.90% as the A/O ratio decreased from 5/1 to 1/5. The extraction percentage of Al(III) was negligible in all the A/O ratio studied. The results in Fig. 3 indicated that two stages are required to obtain a quantitative extraction of Ag(I) using 0.3 mol/L of LIX63 at an A/O ratio of 1/1.
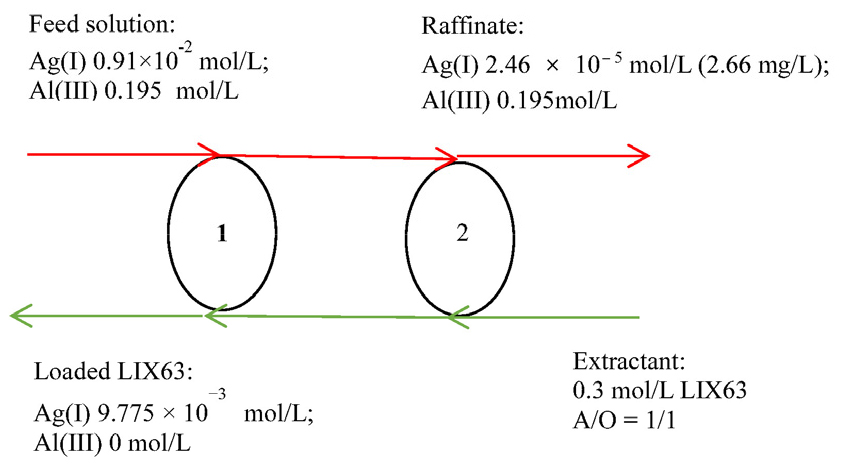
Based on the results obtained from the McCabe–Thiele plot, a two-stage counter-current batch simulation test was performed at an A/O ratio of 1/1 using 0.3 mol/ L of LIX63. The results in Fig. 4 shows that after the two-stage counter-current extraction, 99.75% of Ag(I) was extracted from the aqueous solution, while Al(III) remained in the raffinate. The Ag(I) and Al(III) concentrations in the raffinate were 2.46 × 10-5 mol/ L (2.66 mg/ L) and 0.195 mol/ L, respectively, indicating that the purity of Al(III) was 99.99%. The Ag(I) and Al(III) concentrations in the loaded LIX63 were 9.775 × 10-3 and 0 mol/ L, respectively.
3.4. Stripping of Ag(I) from the loaded LIX63
The experiments for stripping of Ag(I) from the loaded LIX63 were performed using various concentration of ammonia water. The feed solution which contained Ag(I) 0.01 mol/ L, Al(III) 0.195 mol/ L, and HNO3 0.4 mol/L, was used to prepare the loaded LIX63. This feed solution was contacted with 0.5 mol/ L of LIX63, as described in Section 2.3. The loaded LIX63 was contacted with each strippant at an A/O ratio of 1/1. The results (Table 2) indicate that when the concentration of ammonia water increased from 10 to 25%, the stripping percentage of Ag(I) increased from 13.6 to 77.1%. Thus, 20% of ammonia water was selected as the strippant for stripping of Ag(I) from the loaded LIX63. The reaction equation can be represented as follows,
Table 2.
Stripping of Ag(I) from the loaded organic phase with ammonia water
| Agent | Stripping percentage (%) |
| 10% ammonia water | 13.6 |
| 15% ammonia water | 44.8 |
| 20% ammonia water | 76.0 |
| 25% ammonia water | 77.1 |
Where H2R denotes LIX63.
3.5. Effect of phase ratio on the stripping of Ag(I) with ammonia water
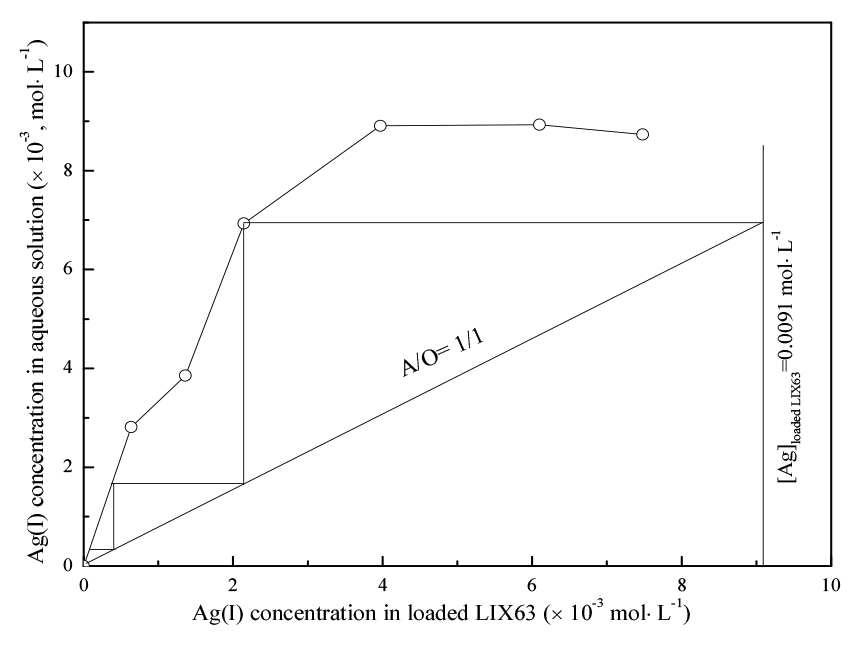
A McCabe–Thiele plot for the stripping of Ag(I) was constructed by varying the A/O ratio from 1/5 to 3/1 to estimate the number of stages and phase ratio to complete the stripping of Ag(I) from the loaded LIX63 with ammonia water. The loaded LIX63 was prepared by extracting Ag(I) from the aqueous solution containing Ag(I) (0.011 mol/ L) and Al(III) (0.195 mol/L) with 0.3 mol/L of LIX63 at 0.4 mol/L of HNO3. Accordingly, 20% of ammonia water was used as the strippant. The Ag(I) and Al(III) concentrations in the loaded LIX63 were 0.0091 and 0 mol/L, respectively. The results showed that the stripping percentage of Ag(I) increased from 18.4 to 96% as the A/O ratio increased from 1/5 to 3/1. The McCabe–Thiele plot for the stripping of Ag(I) indicated that at least three counter-current stripping stages are required for the complete stripping of Ag(I) from the loaded LIX63 at an A/O ratio of 1/1 (Fig. 5). LIX63 can be regenerated after complete stripping of Ag(I) with ammonia water.
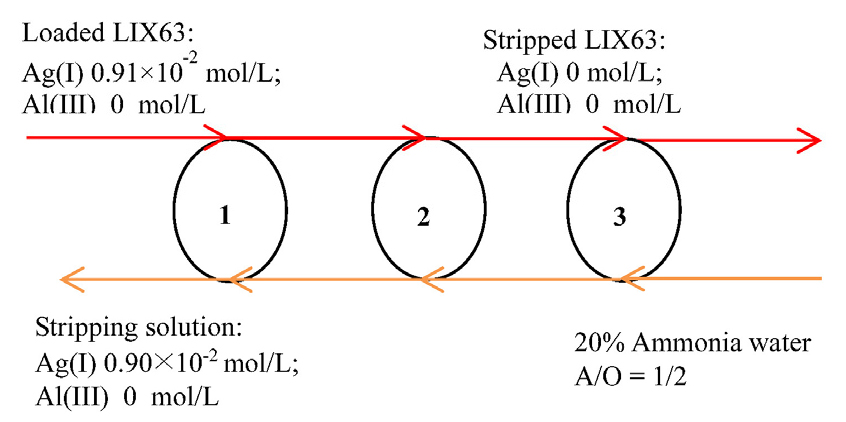
A three-stage counter-current batch simulation of stripping experiments was performed to verify the theoretical stripping stages obtained from the McCabe–Thiele plot. The loaded LIX63 was prepared by extracting Ag(I) from the aqueous solution containing Ag(I) (0.01 mol/L) and Al(III) (0.195 mol/L) with 0.3 mol/L of LIX63 at 0.4 mol/L of HNO3.
The Ag(I) and Al(III) concentrations in the loaded LIX63 were 0.0091 and 0 mol/L, respectively. Accordingly, 20% of ammonia water was used as the strippant, and the A/O ratio was fixed at 1/1. The results of the batch simulation stripping experiments (Fig. 6) showed that 98.9% of Ag(I) was stripped after the three-stage count-current stripping using 20% of ammonia water at an A/O ratio of 1/1. The Ag(I) concentration in the obtained stripping solution was 0.009 mol/ L, while no Al(III) was detected. This result indicated that the purity of Ag(I) was higher than 99.998% (detection limit: 0.01 mg/L).
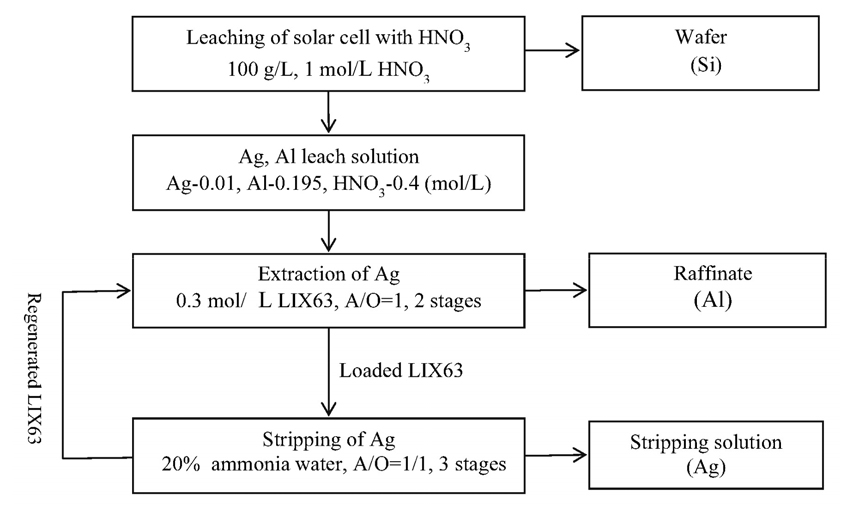
3.6. Process flowsheet for the separation of Ag(I) and Al(III) with LIX63
Fig. 7 shows a process flowsheet for the separation of Ag(I) and Al(III) from the nitrate leach solution of the solar cell developed herein. After leaching the solar cell using the HNO3 solution, Ag(I) and Al(III) are completely dissolved in the leach solution, leaving Si in the solid. The HNO3 concentration in the leach solution was 0.4 mol/L. In the leach solution, Ag(I) was selectively extracted with LIX63 at unit A/O ratio in two stages, leaving Al(III) in the raffinate. The stripping of Ag(I) from the loaded LIX63 was achieved using 20% of ammonia water at an A/O ratio of 1/1 in three stages. The regenerated LIX63 can be re-used in the extraction process after stripping of Ag(I).
4. Conclusions
A solvent extraction process was developed herein to recover Ag(I) from the nitrate leach solution of a solar cell. Ag(I) was extracted selectively with LIX63 and stripped from the loaded LIX63 with ammonia water. The McCabe–Thiele plots for the extraction and stripping were constructed. The number of counter-current stages as well as the volumetric flow ratio of the two phases was obtained. A complete extraction of Ag(I) from the leach solution was managed using 0.3 mol/ L of LIX63 in two stages at an A/O ratio of 1/1, leaving Al(III) in the raffinate. The loaded Ag(I) was recovered by stripping with 20% of ammonia water in three stages at an A/O ratio of 1/1. Finally, this process was verified by the batch simulation experiments of count-current extraction and stripping to obtain Ag(I) and Al(III) solutions with high purity.