1. Introduction
2. Results and discussion
2.1. Chemical reactivity of acids
2.2. Dissolution behavior of individual metals by acid solutions
3. Conclusions
1. Introduction
In hydrometallurgical processes, aqueous lixiviants play a critical role for dissolving metals from ores or concentrates into aqueous solutions. Mineral acid solutions such as H2SO4, HCl, and HNO3 are the most widely used as lixiviants due to their advantages such as aggressiveness, low price and availability1). However, there are some drawbacks in the use of these acidic lixiviants for the leaching of metals from various resources. For instance, the leaching kinetics of copper from sulfidic ore minerals such as chalcopyrite, bornite, chalcocite, and digenite by H2SO4 solution is slow and the formation of passive film on the surface of chalcopyrite like solid sulfur hinders the progress of leaching in the absence of pitting agents like chloride ions2,3,4). Although the leaching rate in chloride media is faster than that of sulfate media due to the high solubility of chloride salts, HCl solution has some disadvantages like the corrosion of the reactors and difficulty of electrowinning of high grade metal5). Due to its highly oxidizing characteristics, nitric acid solutions can oxidize both metals and nonmetal elements like sulfide to sulfur or sulfate6) and produce noxious and corrosive gas products like NOx in the leaching process, resulting in an increase in reagent consumption7,8).
Methanesulfonic acid (CH3SO3H, MSA) has been widely used as an electrolyte for electrochemical processes (especially for tin and lead)9) and can be applied in the synthesis of catalysis10) and polymer11). In recent years, the use of MSA for the leaching of metals in hydrometallurgical processes has attracted many interests due to its excellent chemical and physical properties such as high acidity, solubility of metal salts, high conductivity, high boiling point and less corrosiveness compared to commercially available inorganic acids12). Some studies have pointed out that some metals such as Cu, Ni, Pb, Zn, and rare earth elements can be effectively leached by employing either dilute or concentrated MSA solutions in the presence and absence of oxidizing agents such as H2O2 and ferric chlorides (see Table 1). The leaching efficiency of Cu by the mixture of MSA and H2O2 from chalcopyrite is better than that of sulfuric acid13). Besides, MSA is also known as a green acid owing to no production of dangerous volatile compounds, low toxicity, readily biodegradable14,15). With its characteristics, MSA can be considered as a potential lixiviant for the recovery of valuable metals from the primary and secondary resources.
Table 1.
In this study, the chemical reactivity of MSA was compared with that of sulfuric acid on the basis of analysis of their structure and the dissolution behavior of Co and Ni metal. The dissolution data was obtained from our previously published data16). These obtained results can contribute to provide further information on the application of MSA in hydrometallurgical processes.
2. Results and discussion
2.1. Chemical reactivity of acids
MSA is a strong organic (CH3SO3H, pKa = -1.19) and non-oxidizing acid. The acidity of MSA is close to some inorganic acids such as nitric acid (HNO3, pKa = -1.3) and sulfuric acid (H2SO4, pKa1 = -3) and higher than others such as phosphoric acid (H3PO4, pKa1 = 2.12), acetic acid (CH3COOH, pKa = 4.76) and maleic acid (C2H2(COOH)2, pKa1 =1.83). MSA can be completely ionized into proton and methanesulfonate anions (CH3SO3-) at 0.1 M in aqueous solution as represented in Eq. (1)17). Meanwhile, sulfuric acid is strong inorganic acid with two protons and strong oxidizing agent at concentrated solutions and SO2 gas can be produced at high temperature, which can act as a reducing agent18). The dissociation of sulfuric acid in water depends on solution acidity. When solution acidity is high, the first dissociation of sulfuric acid occurs completely, while the second dissociation can occur in dilute acid solution. The first and second dissociation of sulfuric acid is represented as Eqs. (2) and (3).
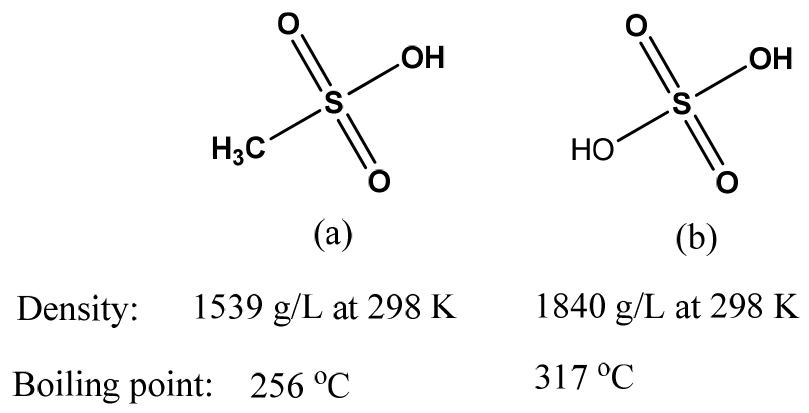
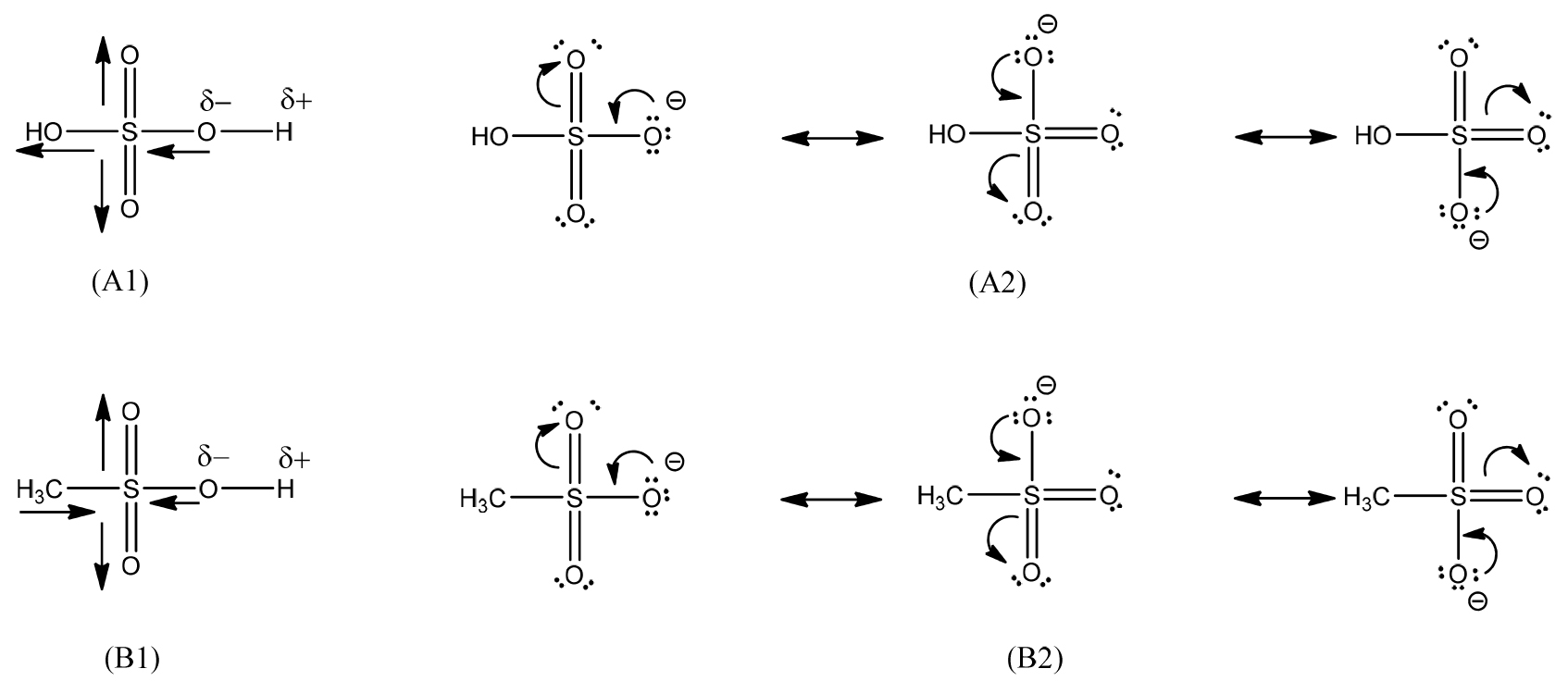
Fig. 1 shows the similarity (-SO2OH group) and difference (CH3- and -OH groups) in the chemical structure of MSA and sulfuric acids, which relates to their chemical reactivity. In their structure, HOSO2- and CH3SO2- groups attached to -OH groups are quite strong electron withdrawing groups owing to the presence of highly electronegative oxygen atoms and the moderately sulfur atom (see Fig. 2. A1&B1). The inductive effect increases the polarity of O-H bonds, resulting in the easy liberation of protons. Besides, resonance effects (see Fig. 2. A2&B2) also contribute to an increase in these polarity. The strong polarity of O-H bonds can explain for the strong acidic properties of sulfuric acid and MSA. The higher acidity of sulfuric acid compared to MSA was ascribed to the positive inductive effect due to electron donating group of methyl group (CH3-) in MSA. Moreover, these inductive and resonance effects lead to the stability of hydrogen sulfate (HSO4-) and methane sulfonate (CH3SO3-) ions due to the stabilization of the negative charge on the oxygen atom after being deprotonated19). This can be shown in the solubility of their metal salts (see Table 2). Most metal methanesulfonates and hydrogen sulfates are highly soluble in aqueous solutions. Only some metal sulfates such as Ca2+, Sr2+, Ba2+, Ag+, and Pb2+ are sparingly soluble in water. On the other hand, some studies have pointed out that MSA aqueous solutions can suppress the oxidation of metal ions to their high valence states13,14). For instance, Sn(II) in the aqueous MSA solution can exist at the lower valence state and the oxidation to Sn(IV) would not occur. The oxidative stability of metal ions in MSA solution is ascribed to the formation of highly stable metal complexes12,24). Thus, MSA will bring many advantages to extractive metallurgy as well as electrochemical processes in the production of metals from their solutions.
Table 2.
The equivalent conductance (S·cm2/mol) of aqueous solutions of sulfuric acid and MSA14)
| Acid | 2 N | 1 N | 0.5 N | 0.1 N | 0.05 N | 0.01 N |
| Sulfuric | 413.84 | 444.88 | 464.12 | 529.08 | 572.76 | 699.40 |
| Methanesulfonic | 232.97 | 299.60 | 336.47 | 372.74 | 381.76 | 391.78 |
2.2. Dissolution behavior of individual metals by acid solutions
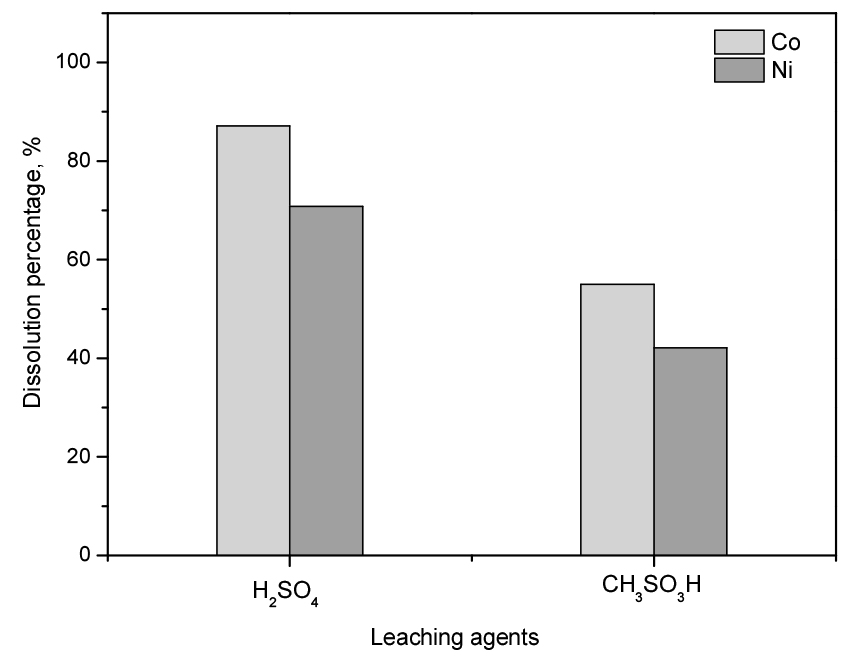
To compare the reactivity of MSA and sulfuric acid in aqueous solutions, the dissolution of Co and Ni metal is compared under the same experimental conditions. The data was obtained from our previous published data16). Concentration of the two acids in the aqueous solutions was fixed at 2.0 mol/L. The experimental leaching conditions were as follows: 60°C temperature, 300 rpm stirring speed, 120 min reaction time, and 50 g/L pulp density. In Fig. 3, the dissolution percentage of Co and Ni by MSA solution were 70.8 and 42.1% and that by H2SO4 solution were 87.1 and 55.0%, respectively. These results showed that the dissolution of metals in sulfuric acid solution was more effective than that of MSA, which can be attributed to the stronger activity of hydrogen ions in sulfuric acid compared to MSA solution. The difference in the effect of the respective anions, CH3SO3- and HSO4- on the dissolution of metals is negligible. This could be due to the insignificant difference in the affinity of two anions to Co(II) and Ni(II) ions, which might be recognized in the solubility of their salts in water (see Table 3). Besides, the dissolution percentage of Co was higher than that of Ni in these acids, which was ascribed to the difference in their nature such as crystalline structure, reduction potentials of metals (= -0.28V and = -0.25V) and stable complexes in the solution. Dissolution reactions of metals can be written as
where M denotes Co or Ni.
Thus, the dissolution efficiency of Co and Ni metal in the MSA and sulfuric acid solutions mainly depends on the redox potentials of the metals and the activity of hydrogen ions in the solutions.
Table 3.
The aqueous saturation solubility of metal salts at 22°C14)
| Metal ions | Methanesulfonate | Sulfate |
|
Li+ Mg2+ Ca2+ Sr2+ Ba2+ Mn2+ Co2+ Ni2+ Cu2+ Ag+ Pb2+ |
7.06 1.40 2.92 2.55 1.59 2.90 2.53 2.13 2.00 3.72 2.60 |
8.17 2.63 0.02 0.00 0.00 3.52 2.16 2.44 1.35 0.06 0.00 |
3. Conclusions
Chemical reactivity between MSA and sulfuric acid was compared on the basis of their structure and dissolution data of metals in their aqueous acidic solutions investigated. The electronic effects such as inductive and resonance have significant contribution for the difference in the chemical properties of these acids such as acidity, solubility of metal salts and affinity to metal ions. The dissolution efficiency of Co and Ni mainly depends on the activity of hydrogen ions in the acid solution, resulting in more effective dissolution by sulfuric acid solution than MSA solution in the same experimental condition. Thus, these data showed MSA can be used as a lixiviant in the recovery of metals from the primary and secondary resources. And, further studies are necessary for the application of MSA in the real operation.