1. Introduction
2. Experimental
2.1. Chemicals and resins
2.2. Ion exchange procedure and analytical methods
3. Results and Discussion
3.1. Removal of Si(IV) from the synthetic sulfate solutions by Diphonix resin
3.2. Removal of Mn(II) from the synthetic chloride solution by P204 resin
4. Conclusions
1. Introduction
Lithium-ion batteries (LIBs) are essential power sources used in advanced electronic devices to provide uninterruptible power and storage1). The growing industrial applications of LIBs lead to increased global production as well as environmental pollution due to improper disposal of used batteries. Therefore, metal recovery from spent LIBs has both economic and environmental value2). Nickel, cobalt, manganese, and lithium are the most important metals present in spent LIBs. The growing demand for these metals leads to rapid depletion of their resources3). The recovery of cobalt and nickel in chloride and sulfate solutions from spent LIBs have attracted much interest.
In our previous work, the combined processes for the recovery of valuable metals from sulfate and chloride solution from spent LIBs were reported4,5). Smelting reduction of spent LIBs at high-temperature results in metallic alloys containing Co, Ni, Cu, Fe, and Mn. Employment of inorganic acid solutions such as H2SO4 and HCl together with H2O2 as an oxidizing agent can completely dissolve these metals from the alloys. The separation of the metal ions such as Co(II), Ni(II), Cu(II), Mn(II), and Fe(III) from the sulfate and chloride leaching solution was carried out by solvent extraction and oxidative precipitation. After these processes, most of the Ni(II) and a small amount of Co(II), Mn(II), and Si(IV) remained in the raffinate. In order to recover pure compounds of these metals, purification of the solutions is necessary.
In the raffinate after solvent extraction, the concentrations of the impure elements are generally very low. Therefore, ion exchange would be efficient for the purification of the raffinate after the solvent extraction. In order to simulate the raffinate from the sulfuric and hydrochloric acid leaching solutions of metallic alloys in our previous work6,7), synthetic sulfate and chloride solutions were employed in this work, whose compositions are shown in Tables 1 and 2. Considering that divalent metal ions exist as cations and Si(IV) exist as anions, cationic exchange resins were employed in this work. Especially, in order to investigate the possibility of separating the divalent metal ions from the solutions, two kinds of cationic exchange resins were employed. Fig. 1 shows that Diphonix is a multifunctional chelating ion-exchange resin with a sulfonic acid cation-exchange group and the phosphonic acid group. The presence of the phosphonic acid group in Diphonix is responsible for the selectivity for some metal cations6). Many works have been reported on the separation of rare earths and base metals from wastewater using P204 (bis(2-ethylhexyl) phosphate)7). Therefore, ion exchange behavior of the ions in the synthetic solutions was investigated by employing Diphonix and P204. Diphonix was effective in removing Si(IV) from the sulfate solutions. P204 resin showed selectivity for the loading of Mn(II) from the chloride solution. The optimum conditions for the loading and elution from the sulfate and chloride solutions were obtained.
Table 1.
The concentration of metal ions in synthetic sulfate solutions of reduction smelted metallic alloys (pH = 3.0)
| Ions | Ni(II) | Co(II) | Mn(II) | Si(IV) |
| mg/L | 1000.0 | 30.0 | 30.0 | 30.0 |
2. Experimental
2.1. Chemicals and resins
The synthetic solutions containing Co(II), Ni(II), Mn(II), and Si(IV) were prepared by dissolving a certain amount of sulfate and chloride salts of the respective metals, such as CoSO4⋅7H2O (Daejung Co., > 99%), NiSO4⋅6H2O (Daejung Co., > 99%), MnSO4⋅4H2O (Duksan Co., > 99%), Na2SiO4 solution (Daejung Co., > 99%), CoCl2⋅6H2O (Junsei Co., > 97%), NiCl2⋅6H2O (Yakuri Co., > 96%), and MnCl2⋅4H2O (Daejung Co., > 98%) in doubly distilled water to the desired concentrations. The concentration of metal ions in the synthetic solutions is shown in Tables 1 and 2. HCl (Daejung Co., 35%), H2SO4 (Daejung Co., 95%), and NaOH (Duksan Co., > 99%) were used to adjust the pH of the synthetic solution. Solutions of NH4Cl (Junsei Co. Ltd, 28%), HCl, and H2SO4 were diluted with doubly distilled water to the desired concentrations. In the current work, resins such as P204 (Eichrom) and Diphonix (Eichrom) were used without any treatment.
2.2. Ion exchange procedure and analytical methods
The pH of aqueous solutions was determined by an Orion Star thermal scientific pH meter (model A221, USA). Batch loading experiments were done by adding proper amount of resin into a 100 mL screwed cap bottle containing the synthetic solutions. The samples were shaken for 6 hours at 300 rpm stirring speed at room temperature in a shaking incubator (HB-201SF, Hanbeak Scientific Co.). The concentration of metals in the feed and in the effluent after filtration was determined by ICP-OES ((Inductively coupled plasma-optical emission spectrometry, Spectro Arcos). The concentration of metals loaded into the resin was obtained by mass balance. The loading percentage (%) was calculated as
where mo and mx represent the mass of metals in the feed and in the effluent.
A glass column (250 × 10 mm) containing 1 g of loaded resin was employed in continuous elution experiments, whereas 250 mm is the length and 10 mm is the diameter of the glass column. The experiments were done at a constant flow rate of 1.5 mL/min using a pump (FMI lab pump, QG20). The effluent was separated into portions of 10 mL and the concentration of metals in each fraction was measured by ICP-OES. The elution percentage was defined as
where and are the mass of metal in the effluent (each 10 mL) and in the loaded resin.
3. Results and Discussion
3.1. Removal of Si(IV) from the synthetic sulfate solutions by Diphonix resin
When the pH of sulfate solution is 3.0, most of the divalent metal ions exist as cation while Si(IV) exists as silicate. In order to investigate the effect of the nature of the resins on the loading of metal ions from sulfate solution with pH 3.0, the experiments were done by using cation exchange resin as Diphonix. The concentration of Ni(II), Co(II), Mn(II), and Si(IV) in sulfate solution was 1000.0 mg/L, 30.0 mg/L, 30.0 mg/L, and 30.0 mg/L, respectively. Table 3 shows the loading percentage of the metal ions in the experiments carried out for 6 h, 20 g/L pulp density, and 300 rpm at room temperature. The loading percentages of Ni(II), Co (II), and Mn(II) on Diphonix resin were 86.6%, 88.0%, and 93.0%, respectively while the loading percentage of Si(IV) was less than 5%. In experiments with cation exchange resin, the loading behavior of Ni(II), Co(II), and Mn(II) was similar to each other. Metal ions show high adsorption capacity on Diphonix resin, indicating that Ni(II), Co(II), and Mn(II) exist as cations in the synthetic sulfate solution. Since the charge of these divalent metal ions is the same and the ionic radius decreases in the order of Mn(II), Co(II), and Ni(II), Mn(II) can be preferentially loaded into Diphonix resin. This might explain the reason why the loading percentage of Mn(II) was slightly higher than that of Co(II) and Ni(II) in these experimental conditions. However, the adsorption behavior of Ni(II), Co(II), and Mn(II) on cation exchange resins was similar. This result demonstrates that most of the divalent metal ions exist as cations while Si(IV) exists as silicate anions in a sulfate solution with pH 3.0. Dissolved silicate exists as ortho-silicic acid (H4SiO4) or Si(OH)4 in aqueous solution and the silicates could bind together to form aggregates when solution pH is less than 3.08). In ion exchange with the Diphonix, some of the silicate aggregates might be formed and then loaded into the cation exchange resin. Therefore, Diphonix was effective in removing Si(IV) from the sulfate solutions. The ion exchange reaction can be expressed as
whereas M2+ represents Ni(II), Co(II), and Mn(II).
Table 3.
The loading percentage of the ions into Diphonix resin from sulfate solution with pH 3.0. (Conditions: 20 g/L pulp density, 6 hours, 300 rpm, room temperature)
| Resin |
Ni(II) (%) |
Co(II) (%) |
Mn(II) (%) |
Si(IV) (%) |
| Diphonix | 86.6 | 88.0 | 93.0 | 4.2 |
In ion exchange, elution of the metal ions from the loaded resin is an important step in the recovery of metal solutions. For this purpose, elution experiments were tested by employing H2SO4, HCl, and NH4Cl solutions with different concentrations for 6 h, 300 rpm, and 0.75 g/L pulp density at room temperature. The elution reaction can be expressed as
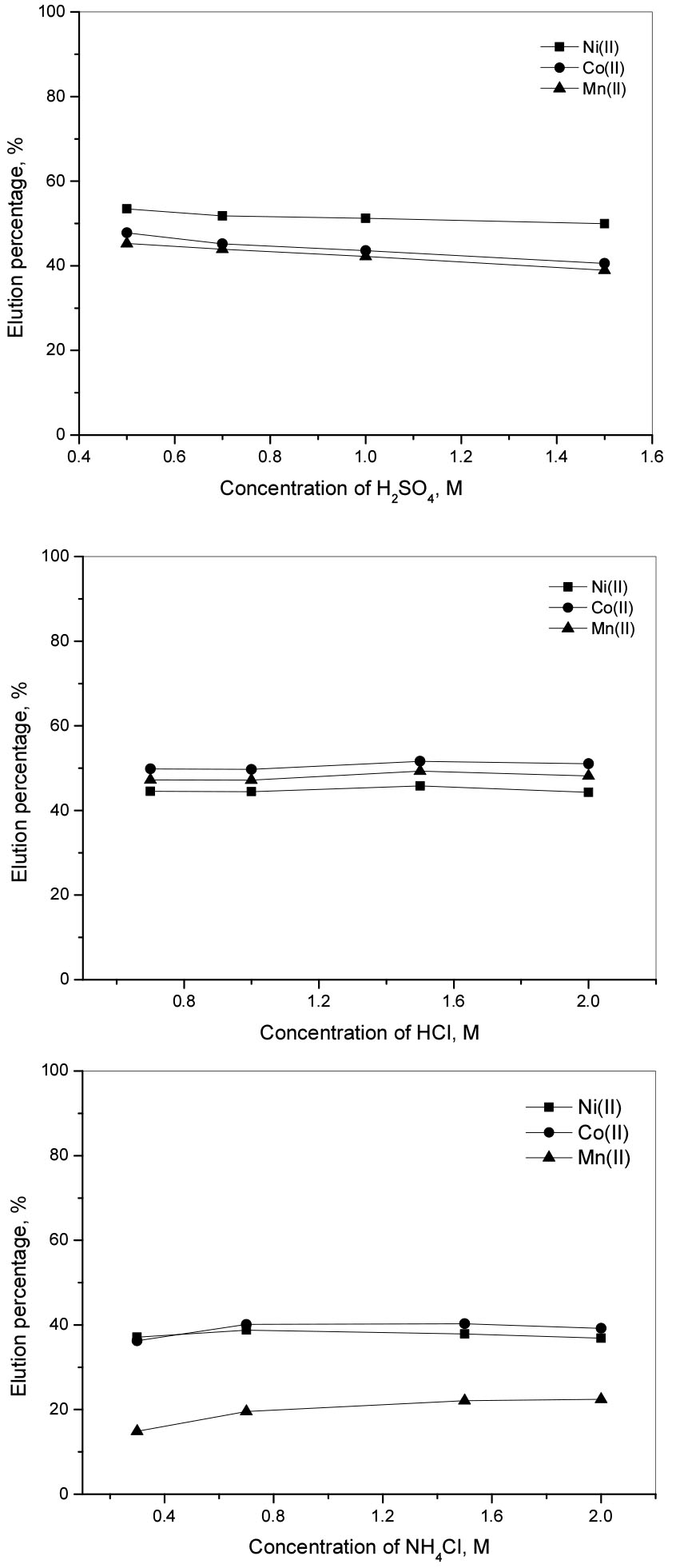
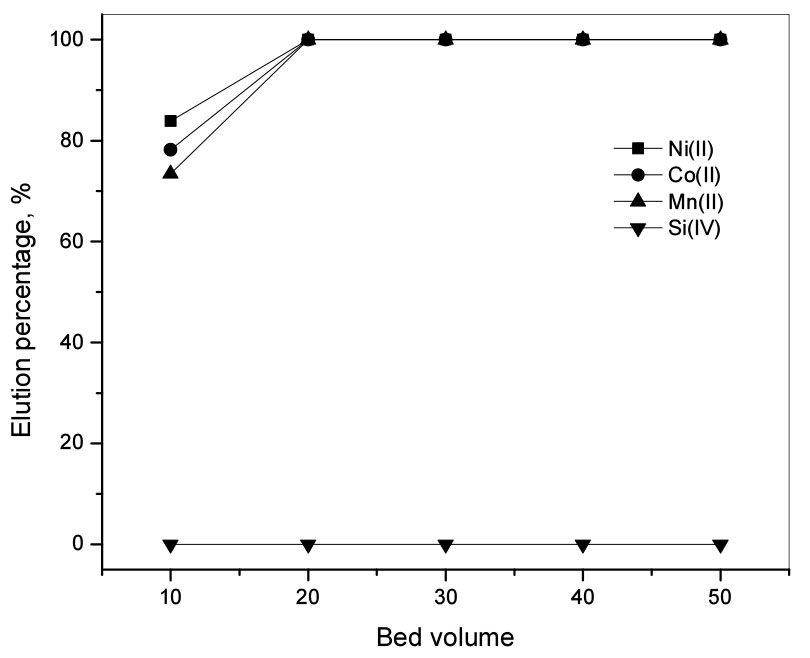
Fig. 2 shows the elution percentage of metal ions with different eluents. When the concentration of H2SO4 solution was increased from 0.5 M to 1.5 M, the elution percentages of Ni(II), Co(II), and Mn(II) decreased slightly from 53% to 50%, from 47% to 40%, and from 45% to 38%, respectively (see Fig. 2). However, when HCl concentration was increased from 0.7 M to 2.0 M, there was little fluctuation in the elution percentage of the metal ions, namely the elution percentages of Ni(II) changed around 52%, Co(II) around 45%, and Mn(II) around 43%. Due to the weak acidity of NH4Cl solution, the elution percentage of the metal ions was lower than that with H2SO4 and HCl solutions. When the concentration of NH4Cl solution was increased from 0.3 M to 2.0 M, 20 % of Mn(II) was eluted while that of Ni(II) and Co(II) was 40%. From the above elution results, 0.5 M H2SO4 solution was chosen for the elution of the metal ions in further experiments. In order to obtain the optimum condition for the continuous elution of the metal ions from Diphonix by using 0.5 M H2SO4 solution, the continuous experiments were done by using 1 g of the loaded resin in the column and the effluent was collected into portions of 10 mL. Fig. 3 shows that 84% of Ni(II), 78% of Co(II), and 73% of Mn(II) were eluted by 0.5 M H2SO4 at 10 bed volume (BV). Because the adsorption tendency of the metal ions onto Diphonix increased in the order Ni(II) < Co(II) < Mn(II), the elution percentage of the metal ions decreased in this order. The plot of cumulative elution of ions from the resin and the bed volume showed that the cumulative elution of ions increased with the increase of the bed volume and was constant after 20 BV (see Fig. 3). Thus it can be concluded the divalent metal ions were completely eluted from the resin at 20 BV of elution with 0.5M H2SO4 and it was possible to separate Si(IV) from the feed.
In the feed solution, the concentration of Ni(II) was about 33 times higher than that of the remaining ions, namely 1000 mg/L Ni(II), 30 mg/L Co(II), 30 mg/L Mn(II) and 30 mg/L Si(IV). Although the adsorption percentage of Ni(II) was lower than that of Co(II) and Mn(II) in the reaction with Diphonix resin, the amount of Ni(II) adsorbed to the resin was still very high. Specifically, 1 g of the resin adsorbed 43.3 mg Ni(II), 1.32 mg Co(II), 1.39 mg Mn(II), and 0.063 mg Si(IV). Therefore, the Ni(II) concentration in the effluent remained high in the different solutions. According to continuous experiment results, a resin concentration of 20 g/L was sufficient to load completely the divalent metal ions from the solution. Moreover, complete elution of these metal ions from the loaded Diphonix resin was carried out by 0.5 M H2SO4 with 1.0 g of the loaded resin in the glass column with 20 BV. By ion exchange with Diphonix, Si(IV) can be completely removed from the solution and the effluent contains 2165 mg/L Ni(II), 66 mg/L Co, and 70 mg/L Mn.
3.2. Removal of Mn(II) from the synthetic chloride solution by P204 resin
Fig. 1 indicates the structure of P204 which is a specific representation of the acidic phosphorus extractants. P204 shows a selective extraction rare earths and iron(III) through cation exchange mechanism7). The extraction reaction of Ni(II) and Mn(II) with P204 can be presented as follows7)
whereas M2+ represents Ni(II) and Mn(II).
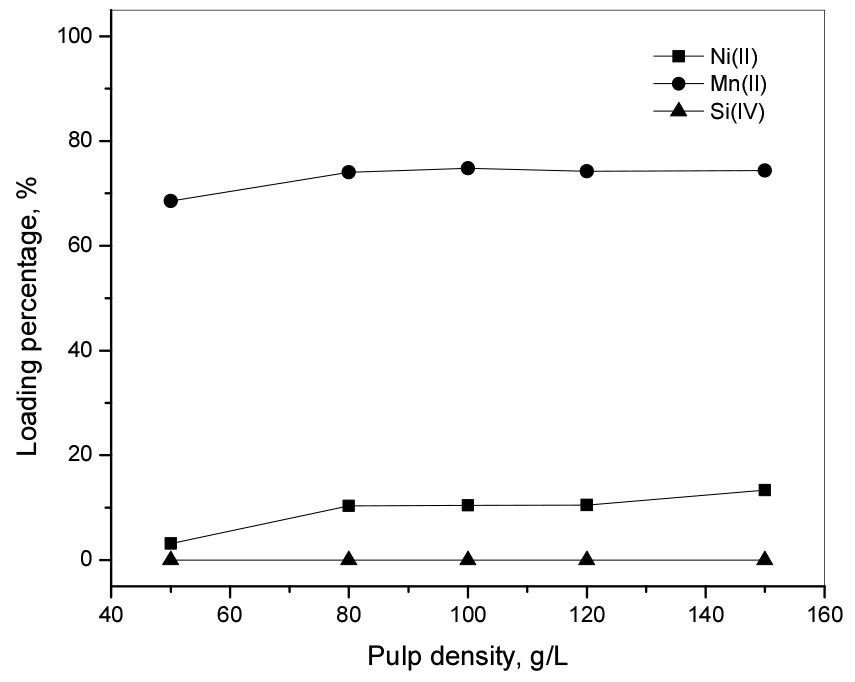
To investigate the loading behavior of the metal ions from the synthetic chloride solutions, pulp density of P204 resin was varied from 50 to 150 g/L. The concentration of Ni(II), Mn(II), and Si(IV) in the solution was 1000 mg/L, 30 mg/L, and 30 mg/L respectively. The initial pH of the solution was fixed at 6.0 and ion exchange experiments were carried out at room temperature for 6 h at a shaking speed of 300 rpm. Fig. 4 shows that the pulp density of P204 resin affected little the loading of Ni(II), Mn(II) and Si(IV). When the pulp density of P204 was 100 g/L, the loading percentage of Mn(II) and Ni(II) was 74% and 10%, while that of Si(IV) was zero (see Fig. 4). In chloride solution, Ni(II) exists as Ni2+ or NiCl+9), and thus it has a lower charge density than Mn(II), which is responsible for the selective loading of Mn(II) on P204 resin. Our results indicate that it is possible to separate Mn(II) from the chloride leaching solution and 100 g/L pulp density of P204 resin was selected for the next experiments.
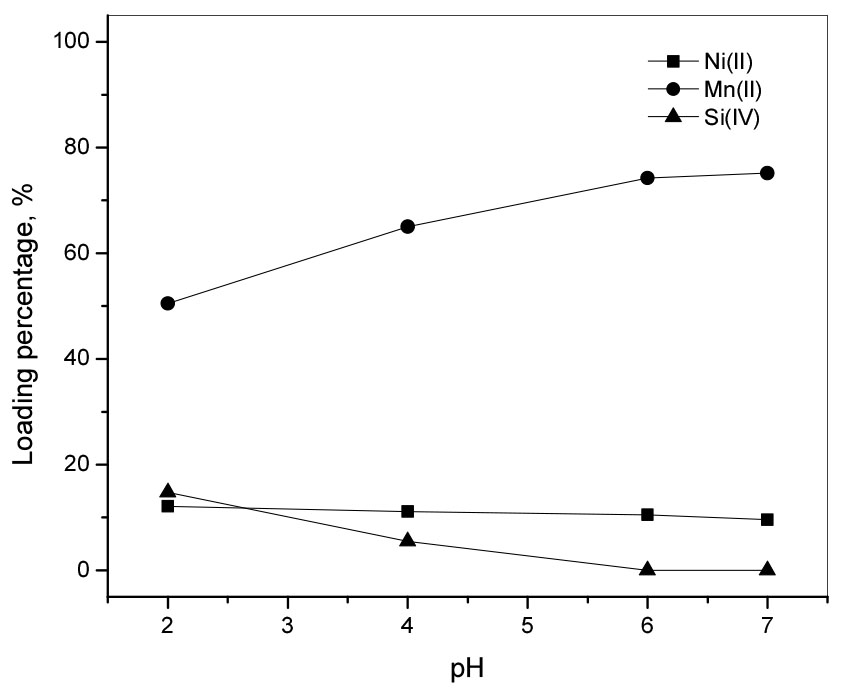
The influence of pH on the adsorption onto P204 resin was investigated by varying solution pH from 2.0 to 7.0 in the synthetic chloride solution. The experiments were performed at a resin concentration of 100 g/L, shaking at 300 rpm for 6 h at room temperature. According to the data in Fig. 5, a small amount of Si(IV) was loaded from the solution with pH 2.0 but Si(IV) was not loaded when solution pH was 6.0 and 7.0. Meanwhile, the loading percentage of Mn(II) gradually increased from 50 to 75% as the pH increased from 2.0 to 7.0 (see Fig. 5). The loading percentage of Ni(II) was rather constant at 10% within the pH ranges. From the loading behavior of Ni(II) and Mn(II) with solution pH, it may be concluded that P204 resin has a selectivity for Mn(II) over Ni(II) and Si(IV). Therefore, control of solution pH is important in separating Mn(II) from the solution.
In order to consider the effect of shaking time on the loading from synthetic chloride solution, shaking time was varied from 3 to 24 h. The solution pH was kept at 6.0 with 100 g/L pulp density and 300 rpm at room temperature. The loading percentages of Mn(II) and Ni(II) fluctuated slightly between 70% and 10%, respectively, while that of Si(IV) was zero (see Fig. 6). These results indicate that the adsorption reaction on P204 resin occurs fast.
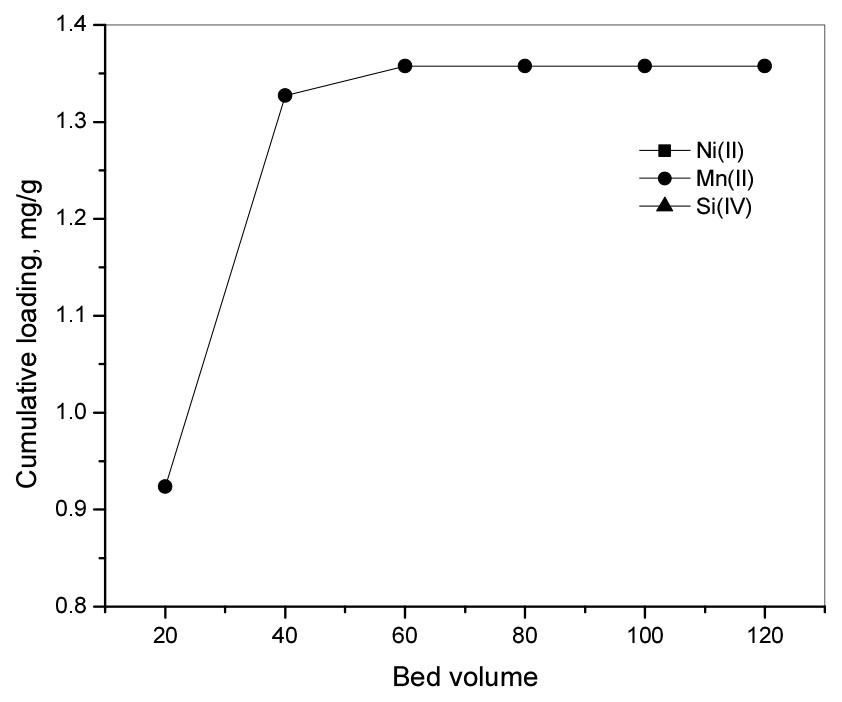
For the separation of Mn(II) from Ni(II) and Si(IV), continuous experiments with P204 were carried out. The plot of cumulative loading of ions in the resin and the bed volume showed that the cumulative loading of Mn(II) increased with the increase of the bed volume and was constant after 60 BV. In this study, 1.35 mg Mn(II) can be loaded into 1.0 g of P204 resin and the loading capacity of P204 for Mn(II) was found to be 0.05 milliequivalent per g of resin. In addition, the loading percentage of Ni(II) and Si(IV) was zero in continuous experiments, indicating that only Mn(II) was loaded into the resin. The obtained results show that Mn(II) can be separated from chloride solution by ion exchange with P204 at pH 6.0.
For the elution experiments of Mn(II), 1g of the loaded resin containing 1.35 mg Mn(II) was prepared by batch experiments. Fig. 8 shows the elution results of Mn(II) from the loaded P204 resin by using 3.0 M HCl solution. The elution reaction can be expressed as7)
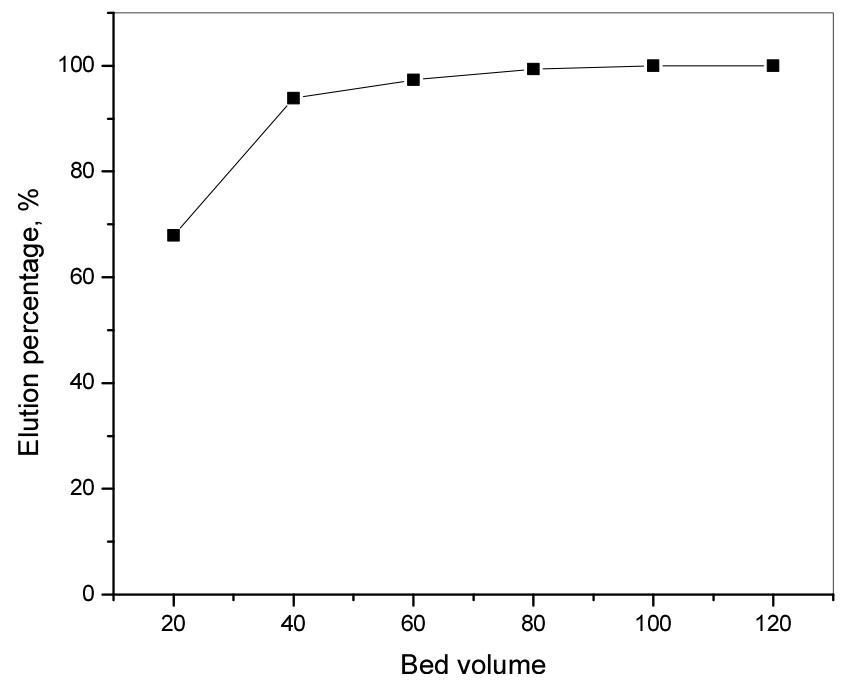
The concentration of Mn(II) in the effluent was declined to zero at 100 BV. Hence, it can be concluded that most of the Mn(II) in loaded resin was completely eluted after 100 BV with 3.0 M HCl and the purity of Mn(II) solution was found about 99.9%.
4. Conclusions
Smelting reduction of spent LIBs at high temperature results in metallic alloys containing valuable metals. In our previous works, two processes were reported to treat these metallic alloys. After leaching the metallic alloys with either HCl or H2SO4 solution, separation steps should be employed to recover pure solutions of the metals. In this work, the ion exchange behavior of Ni(II), Co(II), Mn(II), and Si(IV) from weak sulfate and chloride solutions was investigated. In the case of sulfate solutions containing the above 4 ions, ion exchange with Diphonix was effective in selectively loading Ni(II), Co(II), and Mn(II), while leaving Si(IV) in the effluent. The optimum conditions for the separation of Ni(II), Co(II), and Mn(II) from Si(IV) with Diphonix was 20 g/L pulp density at room temperature in 6h and 300 rpm. The loaded metal ions were completely eluted by using 0.5 M H2SO4 through 20 BV of elution. With chloride solution containing Ni(II), Mn(II), and Si(IV), P204 showed a selective loading of Mn(II) from the feed with pH 6.0. Continuous column experiments verified the complete separation of Mn(II) from the chloride solution in the presence of Ni(II) and Si(IV). The loaded Mn(II) was completely eluted from the P204 by using a 3.0 M HCl solution.