1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Effect of HNO3 concentration on extraction of metals
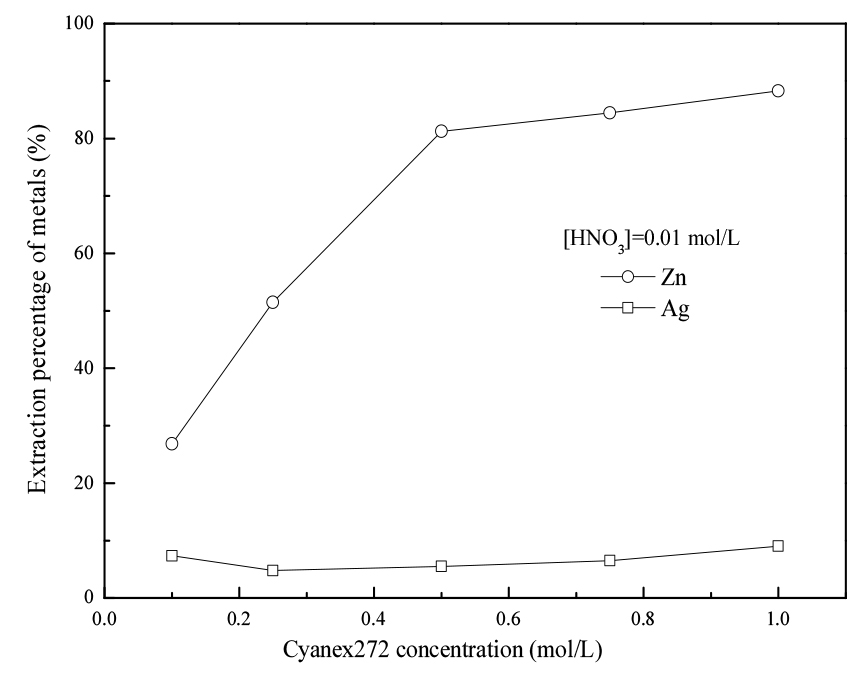
3.2. Effect of cyanex272 concentration on extraction of metals
3.3. Effect of phase ratio on the extraction
3.4. Stripping agent and removal of Ag
3.5. Effect of phase ratio on the stripping of Ag
3.6. Effect of phase ratio on the stripping of Zn after removal of Ag
4. Conclusions
1. Introduction
Silver oxide batteries are applied in a range of electronic products from military applications to medical devices, watches, calculators, and toys1). Spent silver oxide batteries are important resources for Zn and Ag recovery, considering the shortage of this material resource, environmental contamination, and the decline in the cost of waste treatment1,2,3,4).
Among of various recovery process, silver oxide batteries are generally dissolved with nitrate acid, cyanide solution, and fermentation liquor5,6,7). In case of nitrate leachate, Ag and Zn were precipitated by using chloride salts, such as KCl or NaCl8). Expensive equipment and precipitants (including flocculating agent) are inevitable for these methods9). Solvent extraction with various extractants, such as LIX63, D2EHPA, Cyanex471X, were also used to separate Zn and Ag from nitrate leachates of silver oxide batteries10,11,12,13). Compared with other metal separation/recovering methods, solvent extraction has an advantage on easy handing and high process efficiency14,15).
In this study, bis(2,4,4-trimethylpentyl) phosphinic acid (cyanex272) was used to separate Ag and Zn from nitrate leachate of silver oxide batteries. The effects of acid and extractant concentrations, phase ratio on the extraction of them were examined. Stripping agent and phase ratio on the stripping of Ag and Zn was also studied. Simulated count-current extraction and stripping experiments were also performed to validate the results.
2. Materials and Methods
The aqueous solution was prepared by dissolving AgNO3 (99.8%, Daejung Chemicals and Metals Co., Ltd.) and Zn(NO3)2·6H2O (98.0%, Daejung Chemical and Metals Co., Ltd.) in distilled water. For extractant, Cyanex272 was used without further purification. Kerosene (90% distillation at 265 °C; Daejung) was used as the diluent.
In batch extraction experiments, equal volumes (20 mL) of leachate and extractant were mixed in a sealed bottle (100 mL) and shaken for 30 min using a wrist-action shaker (Model 75, Burrell, USA) at ambient temperature. Then the two phases were separated by using a separation funnel. Stripping experiments were performed through the same procedure by mixing the loaded extractant and stripping solution. The concentration of metals in the solution was measured via inductively coupled plasma optical emission spectrometry (ICP-OES; PerkinElmer Optima 8300).
3. Results and Discussions
3.1. Effect of HNO3 concentration on extraction of metals
To investigate the effect of HNO3 concentration on extraction of metal ions, the con-centration of HNO3 in the feed solution was varied from 0.01 to 5 mol/L, the concentrations of Zn and Ag were kept at 0.0015 and 0.0028 mol/L, respectively. The concentration of cyanex272 was maintained at 0.5 mol/L, and the volume ratio of feed solution to extractant was fixed at 1:1.
The results in Fig. 1 show the effect of HNO3 concentration in the feed solution on the extraction percentages of metal ions. The extraction percentage of both of Zn and Ag decreased with increasing the concentration of HNO3 in the feed solution. Cyanex272 selectively extracted Zn over Ag in the HNO3 concentration range lower than 0.1 mol/L. when the concentration of HNO3 was 0.01 mol/L, the extraction percentage of Zn and Ag was 81.25% and 5.5%.
The extraction reaction can be represented as follows,
where (HR)2 denotes cyanex272.
3.2. Effect of cyanex272 concentration on extraction of metals
Effect of cyanex272 concentration on the extraction of metals was investigated by varying the concentration of cyanex272 in the extractant from 0.1 to 1 mol/L. The concentration of Zn, Ag and HNO3 in the feed solution was maintained at 0.0015, 0.0028 and 0.01 mol/L, respectively.
The results in Fig. 2 show that the extraction percentage of Zn increased sharply when increasing the concentration of cyanex272 from 0.1 to 0.5 mol/L then slowed down with further increasing it to 1 mol/L. While the extraction percentage of Ag was lower than 10% in all the studied extractant concentration range. These results indicate a possibility to separate Ag and Zn by extraction with cyanex272.
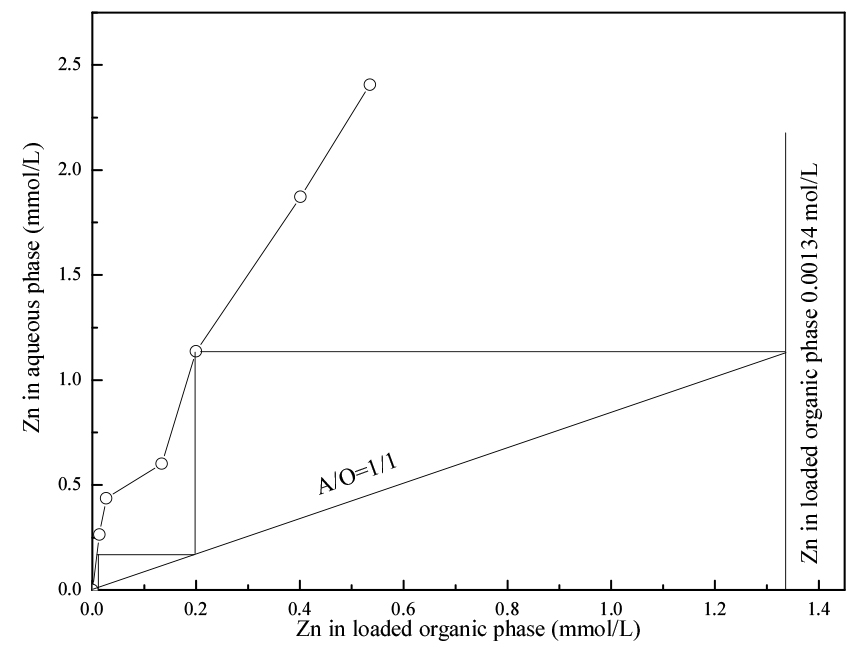
3.3. Effect of phase ratio on the extraction
In order to know the theoretical stages needed for the complete extraction of Zn from the leachate, extraction experiments with 1 mol/L cyanex272 was constructed by varying the volume ratio of extractant to leachate from 1:5 to 5:1. The results are given as the McCabe-Thiele plot for the extraction of Zn. (In the case of Ag, the extraction efficiency was ranged 3~10% and was not given in the figure.) The results in Fig. 3 suggest that 3 stages are needed to completely extract Zn from the leachate with 1 mol/L of cyanex272 at an extractant to leachate ratio of 1.
A two-stage countercurrent batch simulation test was carried out at 1:1 volume ratio of cyanex272 to leachate. For each stage, the initial pH was maintained at 2 (±0.05). The concentration of Zn in the raffinate after three stages extraction was <0.01 mg/L. The concentrations of Ag in the loaded cyanex272 was 0.25 mmol/L (27 mg/L).
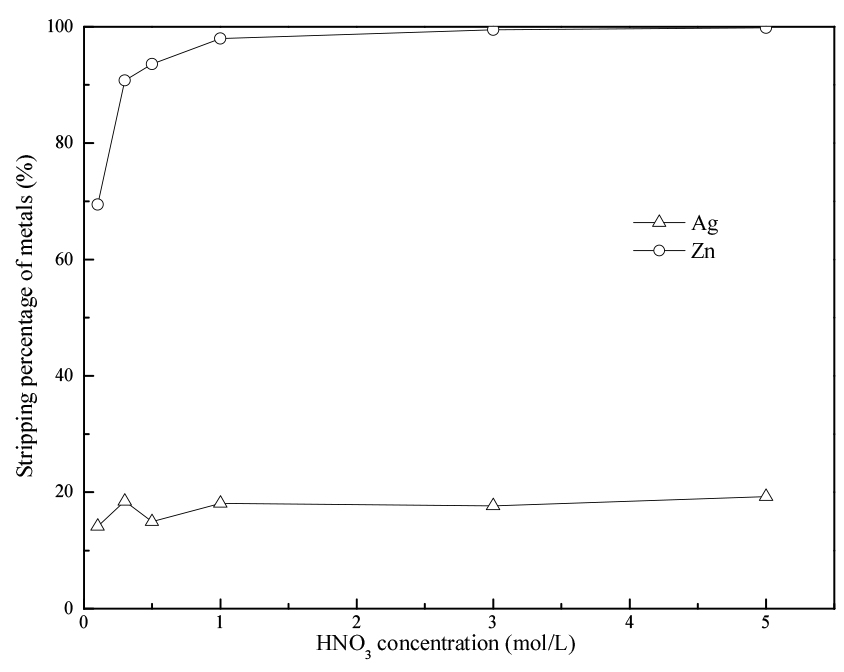
3.4. Stripping agent and removal of Ag
The experiments for removal of Ag from the loaded cyanex272 were carried out by using various concentrations of HNO3 and thiourea. Loaded cyanex272 was prepared by mixing a feed solution containing Zn (0.0015 mol/L), Ag (0.0028 mol/L), and HNO3 (0.01 mol/L), with 1 mol/L cyanex272, as described in Section 2. The loaded cyanex272 was mixed with each strippant at an A/O ratio of 1:1.
The results of stripping of the metals with HNO3 are presented in Fig. 4. The stripping percentage of Zn increased with increasing the concentration of HNO3 from 0.1 to 5 mol/L. In the case of Ag, the stripping percentage increased in a small grade and all the stripping percentages were lower than 20% in the studied HNO3concentration range.
When thiourea was used in the stripping experiments (see Table 1), more than 80% of the Ag loaded in cyanex272 was stripped when the concentration of thiourea was higher than 1 mol/L. While the concentration of Zn was little changed before and after stripping process. Thus, 1 mol/L of thiourea was selected to selectively strip Ag from loaded cyanex272 containing both Zn and Ag.
The stripping reaction can be represented as follows,
where (HR)2 and Th denotes cyanex272 and thiourea, respectively.
Furthermore, based on the results in Figs. 4 and Table 1, the loaded cyanex272 could be regenerated by stripping of Ag with thiourea, followed by stripping of Zn with HNO3 solution.
Table 1.
Stripping of metals from loaded cyanex272 with various concentration of thiourea
| Thiourea concentration (mol/L) | Stripping percentage (%) | |
| Ag | Zn | |
| 0.1 | 15 | 0 |
| 0.5 | 58 | 0 |
| 0.7 | 69 | 0 |
| 1 | 83 | 0 |
3.5. Effect of phase ratio on the stripping of Ag
Effect of phase ratio on the stripping of Ag was investigated by varying the A/O ratio (1 mol/L thiourea to loaded cyanex272) from 1:3 to 2:1. The loaded cyanex272 was obtained by mixing 1 mol/L of cyanex272 and the mixture of Zn and Ag prepared in 0.01 mol/L HNO3. The loaded cyanex272 containing 1.337 mmol/L Zn and 0.227 mmol/L Ag was mixed with 1 mol/L thiourea at varies A/O ratios. A McCabeThiele plot for the stripping of Ag was constructed in Fig. 5. The stripping percentage of Ag was increased from 43% to 90% as the A/O ratio increased from 1:3 to 2:1. The concentration of Zn in the stripping solution was negligible. The results indicate that three counter-current stripping stages are required for the complete stripping of Ag from the loaded cyanex272 at the A/O ratio of 1:1
To verify the above results, a batch simulation stripping experiments were performed. The loaded cyanex272 was obtained by batch simulation experiments of three-stage counter-current extraction, and the concentrations of Ag and Zn were 0.25 mmol/L and 0.0015mol/L, respectively. 1 mol/L thiourea was used as the strippant and the A/O ratio was maintained at 1:1. The results suggested that Ag was completely stripped after the three-stage counter-current stripping, while the concentration of Zn in the stripping solution was negligible.
3.6. Effect of phase ratio on the stripping of Zn after removal of Ag
Effect of phase ratio on the stripping of Zn with HNO3 was investigated by varying the A/O ratio from 1:3 to 5:1. The loaded cyanex272 obtained from the counter-current stripping experiment in part 5 was used. The concentration of Zn in the loaded cyanex272 was 0.0015 mol/L. 0.5 mol/L HNO3 was used as stripping agent.
The results given in Fig. 6 indicate that three counter-current stripping stages are required for the complete stripping of Zn from the loaded cyanex272 at an A/O ratio of 1:1.
A three-stage counter-current batch simulation of stripping experiments of Zn was performed to verify the obtained results. The phase ratio was maintained at 1:1. According to the batch simulation stripping experiments, Zn concentration in the obtained stripping solution was 97.4 mg/L, indicating 99.9% stripping percentage of Zn from the loaded cyanex272.
4. Conclusions
A solvent extraction process was developed for separation of Ag and Zn from the nitrate leachate of silver oxide batteries. The proposed flow sheet is summarized in Fig. 7. Zn and small amount of Ag were extracted with cyanex272 when the concentration of acid in leachate lower than 0.1 mol/L. The co-extracted Ag was removed by stripping with 1 mol/L thiourea. Zn in the loaded cyanex272 after removal of Ag was recovered by stripping with 0.5 mol/L HNO3. Moreover, the feasibility of the proposed process was verified by performing batch simulation counter-current extraction and stripping experiments.