1. Introduction
2. Materials and Methods
2.1. NCM Black mass
2.2. Leaching experiment
2.3. Calculation of metal leaching efficiency
3. Results and Discussion
3.1. Comparison of MSA and sulfuric acid
3.2. Effects of Chemical concentration
3.3. Effects of leaching time
3.4. Effect of leaching temperature
3.5. Effect of Pulp Density
4. Conclusion
1. Introduction
The generation of WEEE (Waste Electrical and Electronic Equipment) has increased due to beginning of information age and international trends of carbon neutrality. The increase of WEEE generation results from demand of electronic devices of which characteristic of short duration1,2,3). Lithium Ion Batteries (LIB) are generally used for the energy source of these EEE. The market size of LIB in 2026 is estimated to be about 139 billion dollar, and the market size will be expected to grow annually4). Especially, the supply of electric vehicles will increase to reduce carbon dioxide emission in transportation due to carbon neutrality in the worldwide. This results in the usage of LIB for electric vehicles, which leads to increase of EOL (End of life) LIB generation. Due to the increase of LIB demand, consumption of the raw materials, lithium (Li), nickel (Ni), cobalt (Co) is expected to increase. This will result in supply chain risk due to scarcity of the minerals. Also, spent LIBs contain cobalt (5 - 20 wt%), lithium (5-7 wt%) and other heavy metals. Environmental pollution of soil and underground water can occur if they are not treated properly. In order to achieve sustainable supply of the critical minerals and environmentally sustainable treatment, recycling of the EOL LIB is essential4,5). Generally, LIB contains cathode, anode, electrolyte and separator6). Among them, cathode affects the performance of LIB. Cathodic materials, especially NCM (Nickel Cobalt Manganese) cathode, consist of metal oxides of Li, Co, Ni and Mn with Al foil7). Recycling of the metals in LIB can be achieved by hydrometallurgical processes. Inorganic acids, such as sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3) are generally used for leaching to dissolve cathodic materials. The acids show high metals recovery, but they are regarded as hazardous materials with high toxicity and corrosiveness and generate soil/water waste containing nitrogen (T-N), which results in environmental risk3,8). Therefore, organic acids has been studied as alternative lixiviants to replace these inorganic acids9,10). Organic acids have their characteristics of low toxicity with biodegradability as they are produced from microbial environment. In addition, they are less corrosive and environmentally sustainable compared to conventional inorganic acid3,11). There are several organic acids such as acetic acid, succinic acid, ascorbic acid, citric acid which have been studied as alternative lixiviant to leach metals from EOL LIB5). However, conventional organic acids show lower acidity and metals solubility compared to inorganic acids, resulting in slower leaching kinetics. MSA (Methanesulfonic Acid, CH3SO3H) is a strong organic acid, which has similar pKa value (pka = -1.9) to conventional inorganic acid8,12,13,14). Also, MSA has high metals solubility with lower toxicity, corrosiveness and environmental impact compared to conventional lixiviant8,15). MSA has been investigated as an alternative lixiviant to leach black mass of LCO (LiCoO2) and LFP (LiFePO4)5,16). MSA was tested to leach LCO black mass and leaching efficiency of Li and Co were reported to be above 99% at 80℃5). LFP black mass was also tested using MSA and p-toluene sulfonic acid (TSA). The study reported that Li leaching efficiency was above 96% when 4 M MSA and 18% H2O2 were tested at S/L ratio of 80 at room temperature16). In this study, MSA was tested to recycle NCM black mass, which targeted to recycle of high nickel battery. This study focused on 1) feasibility study of MSA as an alternative lixiviant and 2) investigation of leaching behavior by different leaching factors such as concentration of lixiviant, reductant, time, temperature, P/D (pulp density) to leach NCM black mass.
2. Materials and Methods
2.1. NCM Black mass
NCM Black mass was collected from one of battery recycling plants in South Korea. Both particle size distribution and elemental analysis were conducted to characterize the collected sample. Based on the results of particle size distribution analysis using multiple sieves, the size of samples for diameter of 50% and 90% passing through were 50 μm and 85 μm, respectively as shown in Fig. 1.
Elemental analysis of the NCM black mass was conducted using ICP-OES (Inductively coupled plasma - optical emission spectrometry). The result shows that the sample contains Li 6.76%, Ni 40.41%, Co 5.77%, Mn 1.44%, Al 1.65%, Cu 6.08% and other elements of 37.89% as shown in Table 1. Based on the elemental composition, the sample seems blended black mass of NCM 811 and other type of cathodic materials such as LCO and NCA (Nickel Cobalt Aluminum). The surface morphology and elemental information was also investigated using SEM-EDS (Scanning Electron Microscopy Energy Dispersive X-ray Spectroscopy).
Table 1.
Elemental composition of NCM black mass sample
| Elements | Li | Ni | Co | Mn | Al | Cu |
| (%) | 6.76 | 40.41 | 5.77 | 1.44 | 1.65 | 6.08 |
Results of SEM image shown in Fig. 2 shows that the size of samples is between 10 and 50 μm and the shapes are heterogeneous. Also, the sample mainly contains oxygen, and nickel with some portion of copper fluoride and cobalt on the surface based on Table 2.
Table 2.
Elemental composition of NCM black mass via SEM-EDS
| Element | Wt (%) | Atomic (%) |
| O | 32.74 | 59.75 |
| F | 5.39 | 8.26 |
| Al | 2.71 | 2.93 |
| Mn | 2.40 | 1.27 |
| Co | 6.55 | 3.25 |
| Ni | 38.22 | 19.02 |
| Cu | 10.40 | 4.28 |
2.2. Leaching experiment
The different concentration of MSA and hydrogen peroxide (H2O2) as a reductant were tested to investigate the leaching behavior on NCM black mass leaching. Concentration of MSA and H2O2 varied from 0.3 M to 2.0 M. In addition, different concentrations of sulfuric acid with hydrogen peroxide were also tested as baseline to leach NCM black mass. The concentration ranges of MSA and sulfuric acid were between 0.3 M and 1.0 M, and the same molarity of the lixiviants were compared. It is because MSA is 1.5 times expensive than sulfuric acid, so it is reasonable to compare leaching efficiency of metals by the same amount of the acids. Meanwhile, the same acidity condition of MSA (1.0 M) and sulfuric acid (0.5 M) were also compared, to compensate the difference of proton generation by each lixiviants. The suite leaching tests were conducted at agitation speed of 300 rpm, 20 g/L pulp density (P/D) and 80℃ during four hours. Then, the different temperature rages were tested at 25, 45, 60, 80℃ to investigate effect of temperature on MSA leaching at 300 rpm during two hours. Finally, various condition of P/D at 20, 50, 80, 100 and 200 g/L at 25℃ with 300 rpm agitation during 4 hours. All the leachate samples were collected at every 1, 2 and 4 hours to investigate the effect of leaching time.
2.3. Calculation of metal leaching efficiency
The collected solution samples were analyzed by ICP-OES. Also, all the leached residue samples were collected and digested using aqua regia to analyze residual composition using ICP-OES. After the analysis of both solution and residue samples, metal leaching efficiency was collected using Eq. (1)17).
In Eq. (1), Rm, Ma and Mb refers metals leaching efficiency (%), mass (g) of target metals at aqueous phase after leaching, mass (g) of target metals at solid phase after leaching, respectively.
3. Results and Discussion
3.1. Comparison of MSA and sulfuric acid
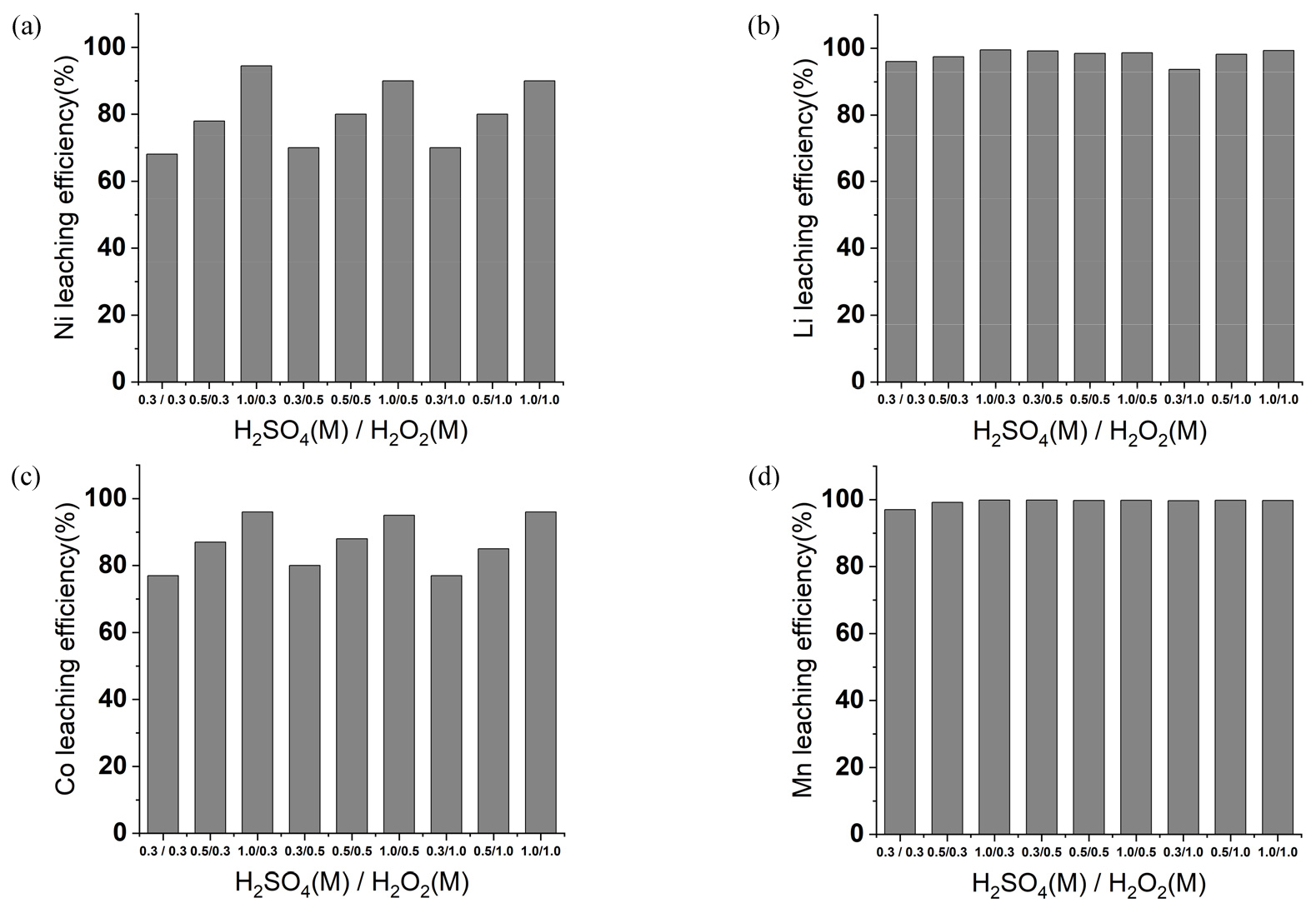
Feasibility study of MSA as an alternative lixiviant on NCM Blackmass was investigated by testing different concentration of MSA and hydrogen peroxide (H2O2) and comparing sulfuric acid (H2SO4) as a conventional lixiviant. The concentration of H2SO4 and MSA were varied between 0.3 and 1.0 M at different H2O2 conditions. As shown in Fig. 3, Ni and Co leaching efficiency after sulfuric acid were between 65 – 90%, and it showed higher metals leaching efficiency above 0.5 M sulfuric acid conditions. In addition, leaching efficiency of Li and Mn was above 95%. At Fig. 4, MSA also showed similar trends, as higher Ni and Co leaching efficiency was observed once concentration of the lixiviant was above 0.5 M. Leaching efficiency range of Ni and Co at the conditions were 77 – 87% and 80 – 90%, respectively. Li and Mn showed leaching efficiency above 95%, which was similar results with H2SO4 – H2O2 leaching. In case of Ni and Co, however, lower leaching efficiency was observed when MSA was used as a lixiviant. Range of Ni and Co leaching efficiency were between 50% and 75% and 60% and 80% by increasing MSA condition from 0.3 M to 1.0 M with fixed 0.3 M hydrogen peroxide. The values were higher with 0.3 – 1.0 M sulfuric acid as Ni leaching of 65% and 94% were observed with 0.3 M and 1.0 M sulfuric acid. Co leaching efficiency with 0.3 M and 1.0 M H2SO4 also showed higher than MSA. Co of 77% was leached with 0.3 M sulfuric acid and it increased to 96% with 1.0 M sulfuric acid. However, leaching efficiency of nickel and cobalt show similar when the same concentration of protons was compared. Sulfuric acid of 0.5 M and MSA of 1.0 M was compared when 0.3 M hydrogen peroxide was used. As mentioned previously, comparison of 0.5 M sulfuric acid and 1.0 M MSA should be also conducted because MSA generates doubled proton than sulfuric acid. The Ni leaching efficiency of 0.5 M sulfuric acid was 78%, and 1.0 M MSA achieved Ni leaching of 77%, which was similar with sulfuric acid. The Co leaching efficiency was also similar as 0.5 M sulfuric acid and 1.0 M MSA showed 88% and 87%, respectively with 0.3 M H2O2 was used. This phenomenon was also observed at fixed hydrogen peroxide condition of 0.5 M and 1.0 M. Especially, 0.5 M sulfuric acid leached Ni and Co about 81% and 87% in each with 1.0 M hydrogen peroxide. Meanwhile, Ni and Co of 87% and 88% were leached respectively with 1.0 M MSA and 1.0 M H2O2. This indicates that MSA showed similar performance with sulfuric acid if the same acidity was compared.
The leach reaction of NCM cathodic materials (in case of NCM 811) with H2SO4 – H2O2 and MSA – H2O2 can be suggested as Eqs. (2) and (3), respectively18).
When NCM 811 was leached with sulfuric acid and hydrogen peroxide, the sulfate to divalent metals (Ni, Co, Mn) ratio of 1:1 can be written as shown in Eq. (2). This means, divalent metal ions form complex with the same amount of sulfate ion. Meanwhile, one mole of methanesulfonic acid generate one mole of proton, and it forms complex with divalent metals such as Ni, Co, Mn with two moles of methanesulfonate anion as shown in Eq. (3). The ratio of methanesulfonate and divalent ions is 2. This can support the result of this study that MSA leaching requires twice higher concentration than sulfuric acid in NCM black mass leaching.
As a result, MSA showed similar trends with sulfuric acid leaching in the presence of H2O2, and it showed feasibility of MSA as an alternative lixiviant to leach NCM black mass.
3.2. Effects of Chemical concentration
3.2.1. Effects of H2O2 concentration
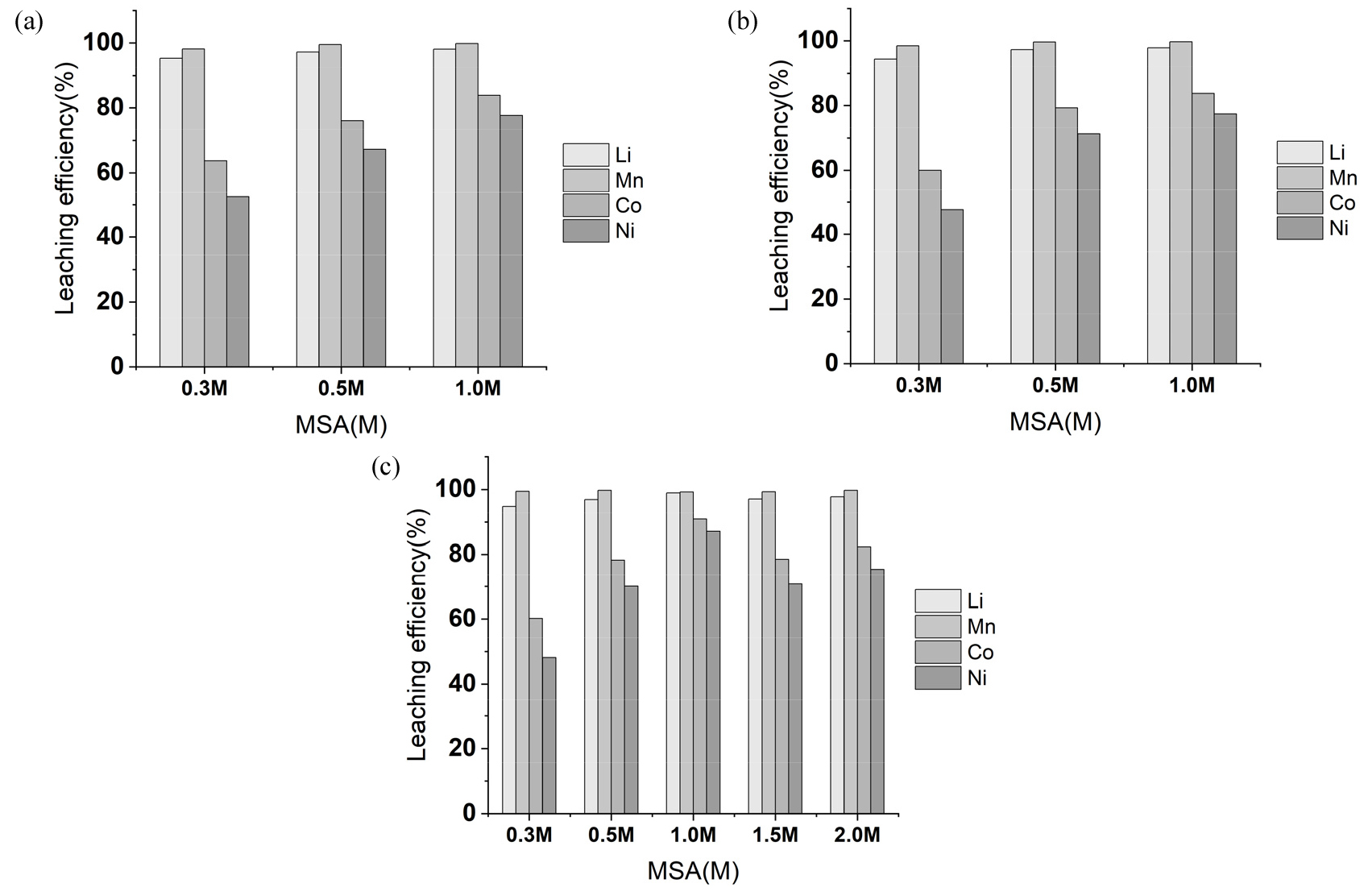
Effects of H2O2 concentration on NCM black mass leaching was investigated at 0.3, 0.5 and 1.0 M MSA as shown in Fig. 5. Leaching efficiency of metals enhanced by increasing concentration of H2O2 from 0.3 to 1.0 M at 0.3 and 0.5 M MSA conditions. Meanwhile, Li and Mn leaching efficiency showed similar regardless of H2O2 concentrations. The value of Li and Mn were about 95% at 0.3 M MSA, but it showed above 98% at 1.0 M MSA, and they were similar at 0.3, 0.5 and 1.0 M H2O2 conditions. It seems that H2O2 concentrations affects more on Ni, Co than Li and Mn. At 1.0 M MSA conditions, recovery of Ni and Co gradually enhanced to 98% once concentration of H2O2 increased from 0.3 to 1.5 M. However, there were no improvement at higher than 1.5 M H2O2 conditions. The concentration of H2O2 influenced more on Ni, Co leaching efficiency rather than Li and Mn in MSA leaching. This can be explained by difference of leaching mechanism of each metal. Leaching of Li from cathodic materials is mainly acid leaching. Lithium in cathodic material stays in monovalent form, which is readily associated to be leached. Therefore, it can be released only by acid leaching. Meanwhile, Mn, Co, Ni are generally stable in oxidized form at cathodic materials, and the reduction of the materials should be conducted. Based on the composition of raw materials, amount of Ni and Co are 38% and 6.5%, which were higher than composition of Mn (2.4%). Therefore, it seems that concentration of hydrogen peroxide affects Ni and Co leaching more than Li and Mn leaching.
3.2.2. Effects of MSA concentration
Fig. 6 shows the effects of MSA concentration on NCM black mass leaching at 0.3, 0.5 and 1.0 M H2O2. At 0.3 M H2O2 condition, Ni and Co leaching efficiency improved from 52.46% to 77.64% and 63.74% to 83.86%, respectively by increasing MSA concentration from 0.3 to 1.0 M. In case of Li and Mn leaching efficiency, above 94% was observed even at 0.3 M MSA. When H2O2 concentration was fixed at 0.5 and 1.0 M, recovery of Li and Mn was similar, but Ni and Co leaching improved by increasing MSA concentration up to 1.0 M. However, metals recovery did not improve when 1.5 M and 2.0 M MSA was tested at 1.0 M H2O2. This means higher than 1.0 M of MSA did not improve the metals recovery at fixed 1.0 M H2O2. This can be explainable by decomposition of hydrogen peroxide at high temperature in the presence of highly acidic condition. It was reported that highly acidic condition triggers decomposition of hydrogen peroxide19,20). Especially, Ni and Co leaching in this test mainly affected by concentration of hydrogen peroxide. As 1.5 and 2.0 M MSA conditions severely acidic condition than below 1.0 M MSA, decomposition of hydrogen peroxide can be affected. It seems that high concentration of protons higher than 1.0 M accelerated decomposition of hydrogen peroxide, which leads to decrease of reducing reaction of nickel and cobalt oxide. This results in lower leaching efficiency of Ni and Co higher than 1.0 M MSA condition.
3.3. Effects of leaching time
The metals recovery at sampling point of 1, 2 and 4 hours were investigated to find the effect of leaching time at 1.5 M MSA and 1.0 M H2O2. As shown in Fig. 7, all the metals achieve the maximum leaching within 1 hours at 25℃. Leaching efficiency of Li and Mn were about 99% and about 70% and 80% of Ni and Co recovery was observed at 1 hours. Then, the metals recovery maintained until the end of leaching test at four hours. Similar leach result was reported when LCO black mass was leached with methanesulfonic acid and hydrogen peroxide. MSA of 1 M and H2O2 of 0.9 vol% leached lithium and cobalt of above 95% within 60 minutes with P/D of 20 g/L at temperature above 70℃5). Also, NCM black mass was leached with 2 M MSA and 0.4 g molasses/g NCM black mass as a reductant and leached above 98% within 60 minutes with pulp density of 50 g/L at 90℃4).
3.4. Effect of leaching temperature
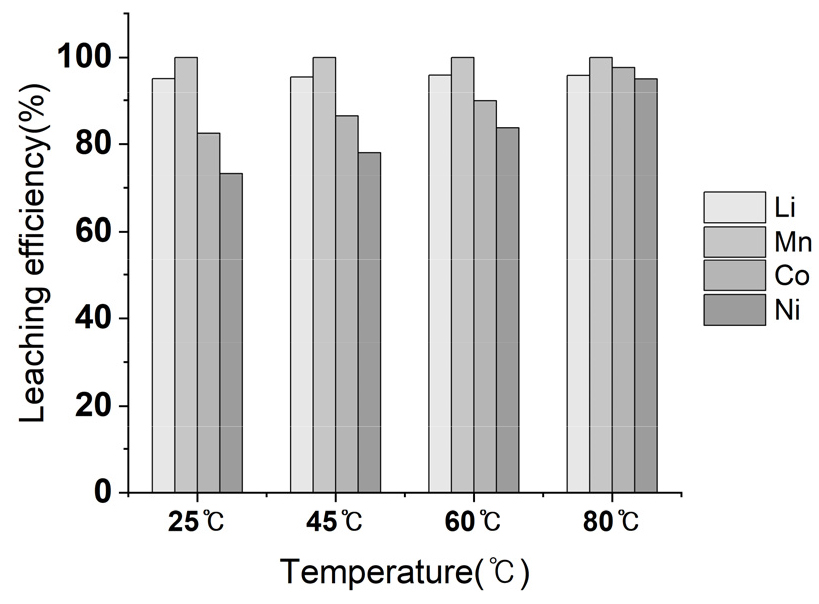
Leaching temperature was varied at 25℃, 45℃, 60℃, 80℃ to investigate the effect of leaching temperature using 1.5 M MSA and 1.0 M H2O2. As shown in Fig. 8, metals recovery of Li and Mn at 2 hours was 95%, 99% respectively. Meanwhile, relatively low Ni and Co leaching efficiency of 80% and 70% was observed at 25℃. By increasing temperature to 80℃, Ni and Co leaching efficiency achieved above 95%. Li and Mn showed similar recovery at 80℃. Based on the result, Ni and Co recovery improved by enhancing temperature from 25℃ to 80℃, but the effect of temperature was relatively minimal on leaching of Li and Mn.
3.5. Effect of Pulp Density
The effect of Pulp Density (P/D) was investigated on 1.5 MSA and 1.0 M H2O2 leaching of NCM black mass at 25℃. Pulp density increased from 20 g/L to 200 g/L and the leach results are shown in Fig. 9. Li leaching efficiency maintained above 95% when the P/D increased from 20 to 100 g/L. It, then, slightly decreased to 90% when NCM black mass was leached for 24 hours at 200 g/L P/D. Leaching efficiency of Mn between 20 and 100 g/L was similar which were close to 99%, but slightly decreased at P/D 200 g/L. Similar trend was observed at Co and Ni leaching. Both Co and Ni recovery were the highest at 20 g/L P/D, showing about 80%. The results of recovery were similar at 50, 80 and 100 g/L. However, dramatic decrease was observed at 200 g/L P/D. Based on the results, increase of P/D affected the recovery of Mn, Co and Ni, especially above P/D of 100 g/L. Meanwhile, recovery of lithium was similar regardless of pulp density. It seems, relatively low leaching efficiency of Co and Ni compared to Mn and Li regardless of P/D. This indicates, the higher amount of chemicals, especially reducing agent should be added in order to achieve higher Co and Ni at high P/D. Therefore, reductant other than hydrogen peroxide can be suggested. A recent study of using molasses as an alternative reductant reported that a glucose from molasses can replace hydrogen peroxide4). It showed higher Ni and Co leaching efficiency above 98% at pulp density of 50 g/L with 1.5 M MSA and reductant dosage of 0.5 g/g. It was higher value of about Ni and Co leaching ratio of 80% in this study. Therefore, in order to achieve higher Ni and Co leaching efficiency in NCM black mass leaching with high P/D, testing of an alternative reductant can be one of the suggestions for future black mass leaching with MSA.
4. Conclusion
Methanesulfonic acid (MSA) was investigated as an alternative lixiviant to replace sulfuric acid for NCM black mass leaching process. The results showed that MSA leached similar metals compared to sulfuric acid by comparing different concentration of the lixiviants with hydrogen peroxide. Therefore, MSA was proven to be an alternative lixiviant to leach NCM black mass. Then, different concentration of MSA and hydrogen peroxide (H2O2) was experimented and increase of both MSA and H2O2 improved metals leaching. The highest leaching efficiency of metals was 1.0 M MSA and 1.5 M H2O2, achieving above 97% within 2 hours at 80℃. This study suggests the feasibility of MSA as an alternative lixiviant to recycle EOL-LIB black mass. Additional studies of leaching kinetics, alternative reductants and compatibility for down streaming processes can be suggested as future studies.